29 Ultrasound Assessment of the Splanchnic (Mesenteric) Arteries
Anatomy, Physiology, and Natural History of Bowel Ischemia
Anatomy
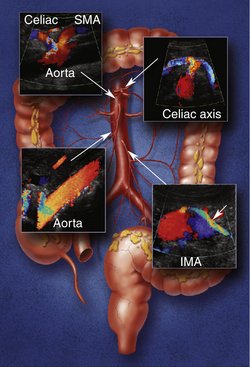
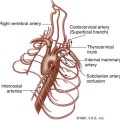
Understanding the mesenteric circulation provides a foundation for the interpretation of the sonographic examination. The splanchnic, or mesenteric, arteries comprise the celiac, superior mesenteric, and inferior mesenteric arteries (Figure 29-1). All three vessels arise from the abdominal aorta. The celiac artery is the first major branch of the abdominal aorta. The SMA is located just inferiorly to the celiac artery. On occasion, the celiac and SMA may share a common origin or trunk. The renal arteries are the next major branches of the abdominal aorta and arise laterally, toward the kidneys. The IMA is identified just below the renal arteries and originates at the left anterolateral aspect of the abdominal aorta. The IMA can be identified in the majority of patients studied for mesenteric insufficiency.
The celiac artery supplies blood to the solid visceral organs (liver, pancreas, and spleen) and the stomach and proximal small bowel. The SMA supplies the bowel from the duodenum to the splenic flexure. The IMA supplies the descending and rectosigmoid colon. A rich collateral network exists between the different mesenteric vessels through the mesenteric arcades and marginal artery. Additional vascular protection is obtained from direct communication between the three mesenteric arteries. Communication between the celiac artery and the SMA occurs by way of the gastroduodenal artery (also known as the pancreatoduodenal arcade; Figure 29-2, A). The superior and inferior mesenteric arteries are connected by the arc of Riolan and the marginal artery of Drummond. The arc of Riolan, also known as the “meandering mesenteric artery” connects the middle colic artery with the left colic artery, and the marginal artery of Drummond connects the ileocolic, right colic, and middle colic branches of the SMA with the left colic and sigmoid branches of the IMA (Figure 29-2, B). In addition, communication also exists between the IMA and branches of the internal iliac arteries. Given this extensive collateral circulation, patients may remain asymptomatic despite the presence of severe mesenteric vascular disease.1–4 In general, severe compromise (>70% stenosis or occlusion) of at least two of the three mesenteric arteries is required for symptoms of mesenteric ischemia to be present. Mesenteric stenosis or occlusion of a single vessel does not usually produce symptoms in light of a patent collateral network. The “two-vessel rule” holds in most patients and is utilized clinically for the diagnosis of chronic mesenteric ischemia.5
Physiology
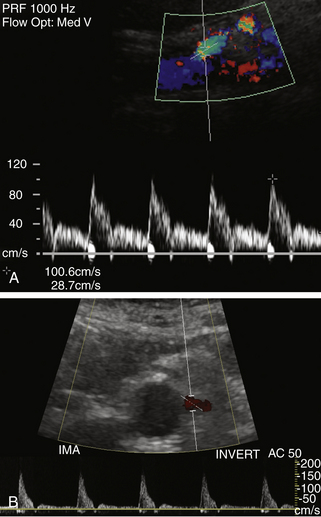
Normal flow patterns differ between the celiac artery and the mesenteric arteries (Figure 29-3). The celiac artery supplies blood to the low-resistance vascular beds of the liver, spleen, and stomach via the hepatic, splenic, and left gastric branches. Pulsed Doppler demonstrates low-resistance flow in the celiac artery, with high end-diastolic velocities (Figure 29-4, A). This low-resistance flow pattern relates to the need for continuous forward flow in both systole and diastole to supply the high oxygen demands of the liver and spleen throughout the cardiac cycle. This flow pattern is similar to the low-resistance signals seen in the renal and internal carotid arteries. The celiac artery low-resistance flow pattern is not dependent on food intake. In other words, there is no significant change in peak systolic or end-diastolic velocities obtained from the celiac artery after a meal.6–9
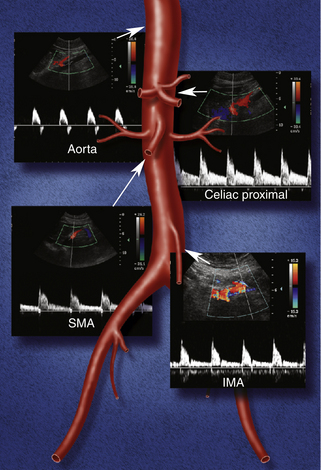
FIGURE 29-3 Montage of normal Doppler waveforms. IMA, inferior mesenteric artery; SMA, superior mesenteric artery.
The superior and inferior mesenteric arteries supply the high-resistance vascular beds of the small intestine and colon. Pulsed Doppler examination reveals high impedance flow with low diastolic velocities in the fasting state (Figure 29-4, B). This is due to the relative vasoconstriction of the splanchnic branch vessels before a meal, when the bowel is empty and quiescent. After a meal, there is an increase in mesenteric arterial blood flow to assist digestion. Vasodilatation of the mesenteric branches allows increased blood flow to the intestines. Moneta and colleagues10 showed that both peak systolic and end-diastolic velocities increase after a meal. The authors described at least doubling of the end-diastolic velocity (EDV) in the SMA after eating. They found the greatest increase in flow after a meal that includes fat, carbohydrate, and protein, and they concluded that this provocative test provides a mechanism for evaluating the reactivity of the splanchnic circulation. The presence of increased flow velocities after a meal was used to infer the adequacy of the splanchnic blood supply in their studies.
Although duplex ultrasound examinations of the mesenteric arteries can be performed before and after a meal to identify physiologic changes in blood flow, there appears to be significant variability in the response to food. Healy and associates11 found that postprandial duplex studies were not dependable and did not improve diagnostic accuracy in their series of patients. We no longer routinely perform preprandial and postprandial duplex examinations of the mesenteric vessels because we found these examinations to be less reliable for the diagnosis of stenosis, as compared with other direct measurements.
Natural History of Bowel Ischemia
Mesenteric ischemia can be classified into four major subcategories according to the cause of vascular compromise. Acute arterial occlusive disease is caused by embolus, thrombosis, or external compression of the vessel. Doppler sonography is generally not useful in this situation because of the rapid time course of the disease and necessity for emergent intervention. Nonobstructive mesenteric arterial insufficiency is the second subcategory and is attributable to hypotensive shock, blood loss, or sepsis that leads to insufficient blood supply to the bowel. Treatment of the underlying condition is key to the management of this type of bowel ischemia. The third category of bowel ischemia is related to venoocclusive disease. Unfortunately, Doppler sonography plays a lesser role in the investigation of this type of bowel ischemia because of the limited ability to detect slow venous blood flow in the main mesenteric and portal veins and their branches. Contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) are more valuable to assess venous patency. The fourth type is chronic mesenteric ischemia (CMI), which in 95% of cases is attributable to atherosclerotic disease causing arterial stenosis or occlusion in the main mesenteric arteries.12 This condition typically occurs in older adult patients with stenosis or occlusion of at least two of the three principal mesenteric vessels (celiac, SMA, and IMA).13 Doppler sonography can play a major role in screening patients with suspected chronic mesenteric ischemia.14–16
Patients with suspected chronic mesenteric ischemia classically present with abdominal pain apparently related to recent ingestion of a meal. Patients typically complain of postprandial pain, bloating, weight loss, and diarrhea. They may describe a “fear of food,” as they experience pain after meals. They may change their diet or eating habits to more frequent, smaller meals to avoid discomfort. In some cases, this change of eating habits is not recognized by the patient, who notes only weight loss. Other patients have a confusing clinical picture with vague symptoms of pain that may or may not be related to meals.17 CMI should be considered in older adult patients with unexplained abdominal pain and weight loss.
Evaluation of the mesenteric arteries is helpful to work through the differential diagnosis in this group of patients with an unclear etiology for their clinical symptoms. Until recently, visceral angiography was the primary diagnostic modality used in the assessment of patients with suspected CMI. With advances in technology, Doppler ultrasound, MR and CT angiography have proven to be accurate noninvasive alternatives to conventional arteriography for the evaluation of mesenteric arteries.18–20 Advantages of Doppler ultrasonography include direct evaluation of all three mesenteric vessels, assessment of the hemodynamics of blood flow, and determination of the significance of lesions involving the visceral vessels. It is an inexpensive, noninvasive method that requires no radiation or contrast material, can be performed portably, and does not have any of the inherent risks associated with other angiographic studies.14 Doppler ultrasound determines the hemodynamic significance of stenotic lesions by demonstrating prestenotic and poststenotic waveform changes. These findings can influence the decision to intervene in the appropriate clinical setting.
Technique
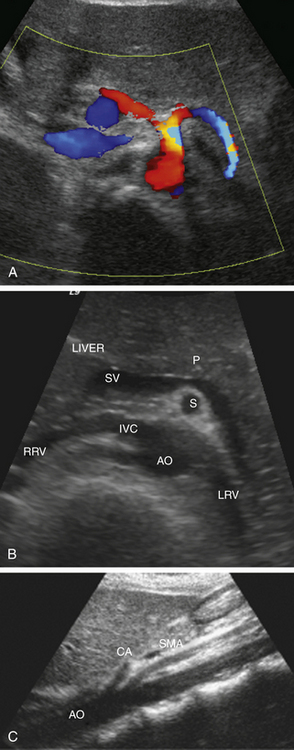
Visualization of the celiac artery origin is initially performed in the sagittal plane. On the transverse view, the branches of the celiac artery have a distinctive appearance with a T-shaped bifurcation (“seagull sign”; Figure 29-5, A). The SMA is seen on the sagittal view arising from the anterior aspect of the aorta, just below the origin of the celiac artery. The SMA serves as a landmark for identification of other major mesenteric vessels because of its unique anatomic location. On the transverse view, it is surrounded by a prominent ring of retroperitoneal fat that separates the SMA from the pancreas. In patients with normal bowel rotation, the SMA lies to the right of the superior mesenteric vein (SMV). The splenic vein and pancreas lie anterior to the SMA, whereas the left renal vein is situated posteriorly. (Figure 29-5, B and C). Visualization of these anatomic structures helps to avoid possible misinterpretation and errors in diagnosis in patients with variant vascular anatomy. One of the most common vascular variants is a separate origin of one of the celiac artery branches arising directly from the aorta or from the SMA. The IMA can be found on the transverse view arising from the anterolateral aspect of the aorta just below the renal arteries.
Several techniques are utilized to optimize assessment of the mesenteric vessels. Harmonic imaging is routinely employed to improve resolution and decrease noise in the image. It has been noted in the literature that studies performed by experienced vascular technologists, sonographers, or sonologists provide the best results.21 Indeed, we have noted that our finest abdominal vascular studies are performed by sonographers and physicians with at least 1 year of experience with abdominal Doppler examinations and a working knowledge of Doppler physics. The learning curve for these examinations depends on the technical ability, motivation, and patience of the examiner. It is clear that a steady volume of abdominal Doppler studies is required to gain proficiency with these examinations. It is also clear that experience with these studies allows the examiner to determine, in short order, whether a successful study will be obtained in any given patient. It should be obvious in most cases whether excessive bowel gas, shortness of breath, large body size, or severe atherosclerotic disease will preclude a complete study. This is usually determined within the first 10 minutes of examination.
The experienced examiner employs a number of techniques to improve visualization of the abdominal vessels and the detection of significant lesions. Optimization of gray-scale and color Doppler parameters assists in the visualization of vessel walls, the detection of atherosclerotic plaque, and assessment of the residual lumen. The color Doppler gain, pulse repetition frequency, and wall filter are adjusted such that laminar flow in the normal segment of the aorta and branch vessel has a homogeneous color flow pattern (Figure 29-6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree