22 Ultrasound Diagnosis of Lower Extremity Venous Thrombosis
Deep venous thrombosis (DVT) is an extremely common medical problem worldwide. The incidence of DVT is estimated to be close to 120 per 100,000 person years,1–3 and recent modeling data suggest that over 900,000 cases of venous thromboembolism occur in the United States per year.3,4 Sequelae of lower extremity DVT include recurrence, postthrombophlebitic syndrome, and chronic venous insufficiency. However, pulmonary embolism (PE) is the most serious consequence of DVT. Fifteen percent of all in-hospital related deaths and up to 20% to 30% of deaths related to pregnancy and delivery are caused by PE with an average case fatality rate estimated to be close to 11%.5 It is estimated that at least 80% of PEs originate from DVT in the lower extremity.6
Lower extremity DVT most commonly originates in the calf veins at the valve leaflets and may extend proximally into the calf and the thigh. The pathophysiology of DVT is best described by the constellation of findings in Virchow’s triad, namely endothelial damage, venous stasis, and hypercoagulable state. Risk factors are listed in Table 22-1 and can be divided into hereditary and acquired causes. The incidence is higher among women than men, higher among African Americans than whites, and lower in Asian and Native Americans.3 The incidence of DVT also increases with age. The most common risk factors include immobilization, pregnancy, major surgery, birth control pills, and hormonal replacement therapies. Because both the age of the population and obesity are increasing in the United States, the incidence of DVT is expected to increase as well.7
TABLE 22-1 Risk Factors for Deep Venous Thrombosis
| Hereditary Factors | Acquired Factors |
|---|---|
| Antithrombin deficiencies | |
| (Protein C and S) | Age |
| Factor V Leiden mutation | Malignancy (advanced) |
| Plasminogen deficiency | Surgery (orthopedic, neurologic) |
| Non-O blood group | Trauma |
| Elevated levels of clotting factors | Immobilization |
| (II, VII, VIII, IX, X, and XI) | Pregnancy and postpartum state |
| Elevated plasminogen activator | Obesity |
| Inhibitor–I | Oral contraceptive use |
| Hyperhomocysteinemia | Hormonal replacement therapy |
| Antiphospholipid antibody syndrome | Hyperviscosity syndromes |
| Chemotherapy | |
| Heparin-induced thrombocytopenia | |
| Myelodysplasia | |
| Polycythemia vera |
The most common signs and symptoms of DVT are lower extremity swelling and pain, particularly if the swelling is unilateral. Bilateral lower extremity swelling is more likely cardiovascular in origin and secondary right to heart failure. A positive Homans’ sign, or pain on forced dorsiflexion of the foot, is considered an unreliable diagnostic criterion. A palpable cord is also an unreliable sign of DVT but is often found in patients with superficial rather than deep venous thrombosis. However, pain and swelling are nonspecific findings, and many patients with DVT are asymptomatic. Therefore, the accuracy of the clinical diagnosis of DVT is extremely poor and estimated at 50%. Compression ultrasound (US) is considered the imaging modality of choice for the diagnosis of DVT. US examination is extremely accurate for the diagnosis of lower extremity DVT in the thigh with reported sensitivities ranging from 88% to 100%, and specificities ranging from 92% to 100%.8–11 Furthermore, US is readily available, inexpensive, noninvasive, and without contraindication or risk. However, US examination is less accurate in detecting calf DVT, where sensitivity is reported to be only 60% to 80%.8,12,13
In many institutions, patients with a high clinical suspicion of DVT or even asymptomatic patients with significant risk factors (prolonged bed rest, major surgery, or malignancy) are immediately evaluated with US examination since it is reliable, safe, and relatively inexpensive. However, despite the efficacy and safety of US for making the diagnosis of DVT, the American Academy of Family Physicians and the American College of Physicians recently published a joint statement recommending that a pretest probability of DVT be estimated before obtaining an US examination.14 The most common means of establishing pretest probability of DVT is with the Wells score (Table 22-2)15 and the D-dimer assay.16–19 The Wells score is based on clinical risk factors. A Wells score less than or equal to 0 is considered low probability; a score of 1 to 2 is consistent with intermediate probability; and a score greater than or equal to 3 is considered high probability for DVT.15 The D-dimer assay measures fibrin degradation products that accumulate in the blood when thrombus forms. If a high resolution D-dimer assay is negative, DVT is very unlikely. Studies have shown that if there is a low pretest probability and a negative D-dimer assay, no treatment or ultrasound examination is necessary.7 However, in patients with a high pretest probability for PE or DVT, the D-dimer assay should not be obtained since the negative predictive value of the assay is low in this clinical situation.5 There are many causes of false positive D-dimer assays, and, therefore, patients should not be treated on the basis of a positive D-dimer assay alone. If the D-dimer assay is positive, an US examination should be performed to evaluate for the presence of DVT. In general, the D-dimer assay is not considered to be helpful or discriminatory in patients who are over 80 years of age, hospitalized, pregnant, or who have cancer since there is frequently a nonspecific elevation of D-dimer levels in these patient subgroups.5,16–19
TABLE 22-2 Wells Scoring for Estimating Pretest Probability of Deep Venous Thrombosis
| +1 Point for Each: |
| Active malignancy (within 6 months or palliative) |
| Paralysis, paresis, or recent plaster immobilization of lower limb |
| Recently bedridden >3 days |
| Major surgery/trauma within past 4 weeks |
| Localized tenderness along distribution of lower extremity veins |
| Swelling of entire lower limb |
| Calf swelling >3 cm compared with asymptomatic leg |
| Pitting edema of symptomatic leg |
| Collateral superficial veins or symptomatic leg |
| −2 Points for: |
| Alternative diagnosis at least as likely as deep vein thrombosis |
| Probability |
| High ≥3 points |
| Intermediate 1-2 points |
| Low ≤0 points |
Technique and Normal Anatomy
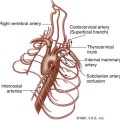
The deep venous system of the lower extremity begins with the three paired calf veins (Figure 22-1). The posterior tibial veins (PTVs) arise posterior to the medial malleolus and ascend posterior to the tibia in the posterior calf muscles. The peroneal veins (PEVs) course laterally in the calf medial and posterior to the fibula. The PTVs and PEVs join in the upper calf to form the tibioperoneal trunk (TPT). The anterior tibial veins (ATVs) drain the anterior compartment of the calf, travel up the calf between the tibia and fibula, and lie anterior to the interosseous membrane. The ATVs join the TPT after crossing the interosseous membrane in the upper calf. The PTVs, PEVs and ATVs are always found adjacent to their corresponding artery and, barring very rare congenital variants, are always paired. Occasionally one or both of the paired veins may be duplicated. Muscular branches within the calf include the gastrocnemius and soleus branches. The soleal branches join either the peroneal or the posterior tibial veins. The gastrocnemius branches join the popliteal above the knee and are typically duplicated and accompanied by gastrocnemius artery.
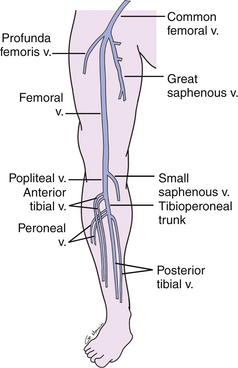
FIGURE 22-1 Schematic drawing demonstrating the anatomy of the deep veins of the lower extremity. v., vein
(Courtesy of Ms. Geri Mancini.)
The TPT and ATVs combine to form the popliteal vein (PV) (see Figure 22-1). The PV courses superficial to the popliteal artery in the popliteal fossa. When the PV enters the adductor canal, it becomes the femoral vein (FV), which travels medial and deep to the superficial femoral artery in the thigh. In the upper thigh, the FV is joined by the deep femoral or profunda femoris vein (PFV) and is then called the common femoral vein (CFV). The CFV runs medial and slightly posterior to the common femoral artery. The external iliac vein (EIV) is the continuation of the CFV above the inguinal ligament. The deep veins of the thigh are duplicated in up to 25% of patients.20 Duplication may be segmental or unilateral. It is advisable to describe duplicated segments in dictated reports so that thrombosis of one of the duplicated veins is not missed on follow-up studies. Unless duplicated, the deep veins of the thigh are generally larger than the accompanying artery and in all cases should run parallel and immediately adjacent to the accompanying artery. The term femoral vein should be used instead of superficial femoral vein in order to avoid confusion in distinguishing the deep from the superficial venous systems. The great saphenous vein (GSV), a superficial vein, joins the CFV medially just above its bifurcation into the FV and PFV.
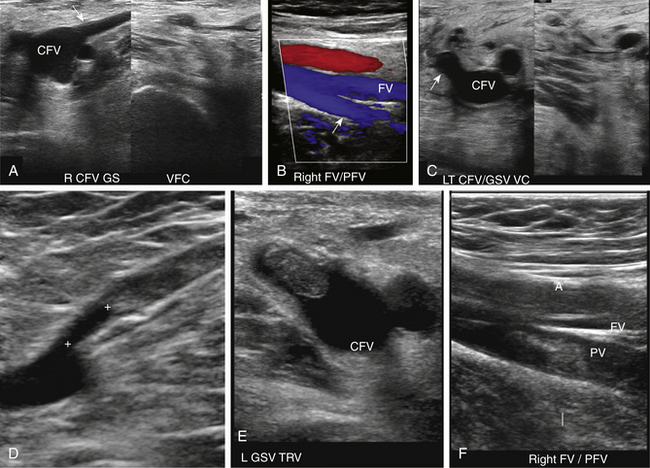
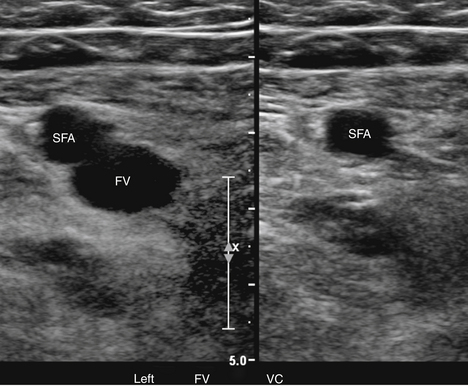
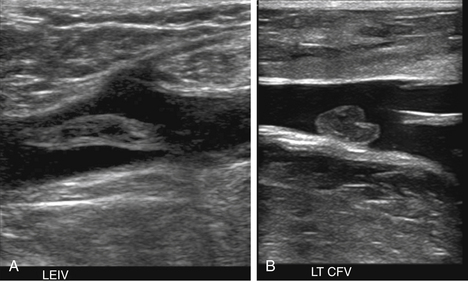
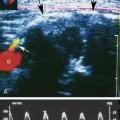
At a minimum, the deep venous system should be examined from the level of the inguinal ligament (CFV) to the tibioperoneal trunk. The CFV and FV are examined with the patient supine and the leg slightly externally rotated. The PV is best evaluated with the knee flexed 20 to 30 degrees and externally rotated (frog’s-leg position) or in the decubitus position. Examination of the proximal GSV, the saphenofemoral junction (SFJ), and the origin of the PFV should also be included according to the practice guidelines recommended by the American College of Radiology (Figure 22-2).21 If desired, the examination may be extended to include the lower EIVs and the calf veins (see later). The CFV, FV, and PV, as well as the origins of the GSV and PFV, should be examined in the transverse plane with and without compression every 2 to 3 cm. With compression, the normal vein wall should completely coapt with obliteration of the vessel lumen (Figure 22-3; see Figure 22-2, C). How much compression is adequate or necessary? In general, if the degree of compression is enough to deform the adjacent artery, it should be adequate to coapt the walls of a normal vein. Compression of the deep veins of the lower extremity may be difficult in the obese or edematous patient, particularly in the adductor canal. In the adductor canal, a two-handed technique may be necessary to demonstrate complete compression of the femoral vein. The examiner places his/her free hand behind the patient’s leg in the adductor canal and pushes the soft tissue toward the transducer until complete venous compression is visualized and documented. Although compression US examination is the key to identification of DVT, some authors advocate avoiding compression in the setting of clearly visualized acute thrombus, particularly if nonadherent or free floating, because of the theoretic risk for embolization (Figure 22-4). However, despite concern for potential embolization of a partially occlusive or free-floating clot, one must be certain that luminal echoes represent DVT and not slow-flowing, echogenic blood. In this case, a single compression event may be sufficient for clarification.
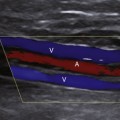
Compression maneuvers should be performed with gray-scale imaging, and it is completely adequate to perform the examination of the venous system to exclude DVT with gray-scale imaging alone. However, color or power Doppler imaging, particularly in the longitudinal plane, may be useful to quickly identify the absence of flow due to an occlusive thrombus or a color void due to partially occlusive thrombus. In addition, color flow imaging may be helpful in examining the obese patient to identify venous structures and demonstrate blood flow signals following the augmentation maneuver when spontaneous flow cannot be demonstrated because of problems with depth penetration and resolution.
Consensus regarding the necessity for evaluation of the calf veins and optimal imaging protocol has not been reached. In 2007, the American College of Chest Physicians’ published guidelines recommended treatment of DVT regardless of location,22 and in accordance that same year, the Intersocietal Commission for the Accreditation of Vascular Laboratories changed their standard to require evaluation of the calf veins.23 While the American Institute of Ultrasound in Medicine (AIUM)/ American College of Radiology (ACR) DVT practice guideline does not require routine calf vein evaluation, AIUM/ACR does require evaluation of the veins of the calf in the patient with calf pain.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree