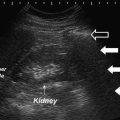
Fig. 11.1
Transrectal ultrasound image (7.5 MHz) of the prostate. Hypoechoic region anteriorly corresponds to transition zone (white arrows). Peripheral zone (relatively more hyperechoic) lies posteriorly (open arrows)
Technique Preparation
Patients undergoing a prostate needle biopsy should refrain from taking antiplatelet/anticoagulation medications (i.e., ASA/NSAIDs/clopidogrel/warfarin) 7 days prior to the procedure. Other medications including over-the-counter medications and herbal products which may affect clotting time should also be avoided. A list of medications which should be avoided prior to biopsy is included in the Appendix.
Crawford et al. [6] have demonstrated that antibiotics 24 h prior to and continuing 24–48 h postprocedure reduce bacterial septicemia. Recently, there has been a rise in septicemia rates from the modern-day prophylaxis rate of less than 1 % [7]. This has been due in part to an increased incidence of extended-spectrum beta-lactamase-producing (ESBL) Escherichia coli that tend to be resistant to ciprofloxacin, ceftriaxone, sulbactam/ampicillin, and cefazolin. Generally imipenem and piperacillin–tazobactam are the effective agents against ESBL-producing E. coli [8, 9]. Prophylaxis against infective endocarditis, with appropriate antibiotics—usually gentamicin and ampicillin [10]—is recommended by the American Heart Association for patients at high risk for infective endocarditis. This includes patients (1) with prosthetic cardiac valves or prosthetic material used for cardiac valve repair, (2) those with previous infective endocarditis, (3) patients with congenital heart defects, and (4) cardiac transplant recipients with valve regurgitation [11]. The American Academy of Orthopedic Surgeons (AAOS) guidelines [12] state that clinicians should consider antibiotic prophylaxis for all total joint replacement patients prior to any invasive procedure that may cause bacteremia. For genitourinary procedures, the AAOS recommends ciprofloxacin 60 min prior to the biopsy. Prophylactic antibiotic use may need to be modified based on local patterns of bacterial resistance.
An enema may be given the night prior to and the morning of the procedure. Reduced rectal contents will increase visibility by reducing interference and have been demonstrated to reduce the rate of bacteremia [13].
Anesthesia
It is accepted that transrectal ultrasound prostate biopsy can be painful [14, 15]. Patient perception of this procedure is a major source of anxiety and a deterrent for undergoing biopsy. Nijs demonstrated 18 % of patients in a population-based screening program refused biopsy due to anticipated pain [16]. Nash first conducted randomized, double-blind studies evaluating the effect of infiltration of 1 % lidocaine in the vascular pedicles of 64 patients undergoing PNB [17]. The mean pain scores on the side injected with drug were significantly lower than the control side. Numerous investigators have demonstrated decreased pain with various preprocedural periprostatic local anesthetic strategies (including a meta-analysis of 14 studies examining 994 procedures) [17–20]. Conversely, a small number of studies have shown no benefit [21–23]. There have been several different periprostatic injection techniques described. The most common strategy involves injection of local anesthetic between the base of the prostate and the seminal vesicles, causing a wheal between the corresponding seminal vesicle and the prostate gland from the rectal wall [24–26]. Other authors have described injecting the prostatic plexus in the area of the apex of the prostate [18, 27]. A majority of studies advocate bilateral injections [19, 24–26]. The administered anesthetic described varies from 1 to 2 % lidocaine [17, 18, 22, 25] with a meta-analysis showing cumulative anesthetic dose varying from 2.5 to 20 mL [20] (Fig. 11.2).


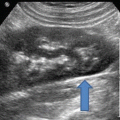
Fig. 11.2
Transrectal ultrasound image (7.5 MHz) of the prostate—sagittal view. Hypoechoic region posterior to the gland represents local anesthetic injection site (arrows)
Transrectal Biopsy Technique
Patients are typically placed in the left lateral decubitus position. Digital rectal exam is performed, and any palpable lesions are noted with respect to their location in the gland. Usually a 7.5 MHz probe is used. The probe is activated and placed using the ultrasound image to guide the probe gently beyond the anal sphincter and adjacent to the prostate. It is recommended to perform this slowly and carefully to minimize patient discomfort. TRUS should be performed in the sagittal and the transverse planes using gray-scale ultrasound. The gland is then inspected using color and power Doppler in both planes for the presence of lesions demonstrating increased flow with respect to other PZ areas of the gland. Abnormalities are recorded by image-saving mechanisms (either paper hard copy or electronic). Localization of all lesions is performed in real time with image documentation. Following instillation of local anesthesia, prostate volume calculations should be carried out. These volumes can be calculated through a variety of formulas that assume the prostate to be that of a geometric shape. These formulas estimate weight as well as volume, as 1 cm3 equals 1 g of prostatic tissue [28]. The specific gravity of prostate tissue is approximately 1.02 g/cm3.
Formulas for estimated weight and volume of the prostate gland:
- (a)
Ellipse: (π/6 × transverse diameter × AP diameter × longitudinal diameter)
- (b)
Sphere: (π/6 × transverse diameter3)
- (c)
Prolate (egg shaped): (π/6 × transverse diameter2 × AP diameter)
Volume measurements of the TZ and bladder volume may be carried out and recorded depending on the preference of the physician. The integrity of the surrounding structures should be evaluated which include examination of the bladder wall, seminal vesicles, and anal canal up to the level of the prostate.
PSA Density
Calculating prostate volume allows the use of PSA density (PSAD ) defined as the ratio of serum PSA-to-prostate volume. PSAD is thought to improve cancer detection (sensitivity) and reduce the number of unnecessary prostate needle biopsies (PNB) (specificity). Djavan and colleagues demonstrated that PSAD and TZ PSAD were significantly higher in subjects diagnosed with prostate cancer on initial and repeat biopsies [29]. The authors routinely calculate PSAD and record it in real time; however, their data do not support basing the decision to perform prostate biopsy solely on this parameter.
Prostatic and Paraprostatic Cysts
As the prostate is examined focal cystic areas can often be noted. These cysts can vary in size and, when associated with BPH , are due to cystic dilatation of TZ glands. Other common cysts include acquired prostatic retention cysts which represent dilatation of glandular acini. Acquired prostatic retention cysts may occur in any zone and are not associated with BPH. Other cysts are less common but have important associations or implications. Utricular and Mullerian duct cysts are congenital midline or paramedian cysts. Utricular cysts are intraprostatic in nature. Arising from a dilated utricle originating at the verumontanum, these communicate with the urethra and can be associated with cryptorchidism and hypospadias [30]. Mullerian duct cysts are located retrovesically and originate from Mullerian remnants. They have no communication with the urethra and can be associated with calculi and renal agenesis [31]. Ejaculatory cysts, arising from an obstructed ejaculatory duct, can be located in a midline or paramedian position. These can be associated with seminal vesicle obstruction and may contain calculi. Seminal vesicle cysts are found lateral to the prostate and are secondary to congenital hypoplasia of the ejaculatory duct [32]. Unilateral in nature, they may contain calculi and are associated with renal agenesis and epididymitis. Prostatic abscesses can be associated with surgery, prostatitis, or epididymitis [33].
Hypoechoic Lesions
The gland should be examined for hypoechoic lesions . The classic appearance of prostate cancer is a round/oval hypoechoic lesion located in the PZ. Contemporary series have noted that the presence of these lesions is a less sensitive sign for prostate cancer than once thought, with hypoechoic lesions being malignant at a rate of 17–57 % [34]. However, a continued valuable asset of TRUS is directed biopsy of these lesions. Absence of hypoechoic lesions is not a contraindication to biopsy as 39 % and 1 % of prostate cancer are isoechoic and hyperechoic, respectively [35]. TRUS has a known poor specificity in regard to the presence of a hypoechoic lesion. Entities which also have a hypoechoic appearance on TRUS include granulomatous prostatitis [36], prostatic infarction [37], lymphoma [38], and TZ BPH [39] (Fig. 11.3).


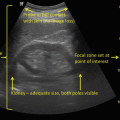
Fig. 11.3
Transrectal ultrasound image (7.5 MHz) of the prostate—transverse view (left) and sagittal view (right). Hypoechoic lesion in the peripheral zone posterolaterally represents an area of increased suspicion for malignancy (arrows)
Color Doppler
Color Doppler (CD) is a tool that attempts to allow TRUS to differentiate benign from malignant tissue. CD measures the frequency shift in sound waves as a measurement of the velocity of blood flow. This capitalizes on the hypervascular appearance of prostate cancer due to increased microvessel density secondary to increased angiogenesis versus benign tissue [40]. Cornud et al. [41] noted that in patients with clinical T1c disease, high-risk pathologic features (ECE, pT3b) were present more often in tumors visualized with CD versus those with the absence of a positive CD signal. Another study demonstrated a 2.6 times increased detection of prostate cancer versus conventional gray-scale ultrasound [34]. Halpern and Strup [42] demonstrated CD sensitivity and specificity to diagnose prostate cancer at 27.3 and 83.9 %, respectively. This is an improvement in specificity with respect to gray-scale imaging (sensitivity 44.4 %, specificity 70.5 %). However, 45 % of cancers still went undetected by any ultrasound modality. Arger [43] has suggested that pathologic groups based on Gleason scores, high (8–10), intermediate (5–7), and low (2–4), were not separable by vascular measurement. While several studies [41, 44, 45] have demonstrated increased cancer detection using CD-targeted focal biopsy strategies, there remain enough questions to preclude replacement of a systemic biopsy approach [46] (Fig. 11.4).


Fig. 11.4
Transrectal ultrasound image, color Doppler, of the prostate—sagittal view. Colored area (arrows) in the posterior lateral aspect of the gland represents an area of increased vascular flow relative to the surrounding parenchyma
Biopsy Strategies
The advent of the sextant biopsy techniqu e, that is, systematic biopsy of the apex, mid, and base of the prostate on each side of the gland, represented an improvement in prostate cancer detection over site-specific biopsies of hypoechoic lesions or palpable abnormalities [5]. With this limited template, there is still concern for a high false-negative rate, with Levine et al. [47] demonstrating in a repeat biopsy series a false-negative rate of 30 % of men with an abnormal digital rectal examination (DRE) and/or elevated serum PSA. Refinements have focused on the importance of increased number of cores, as well as including laterally directed biopsies. Many groups have reported series (Table 11.1) in which improved cancer detection rates are achieved by including additional laterally directed cores and/or increasing the cores taken from 6 to as many as 13 [47–51].
Investigators [52, 53] have demonstrated a lack of usefulness for TZ and SV sampling on initial biopsy with only 2.1 and 3.7 %, respectively, of biopsies being positive. The spatial distribution of cancer foci of prostate cancers with negative initial biopsies or with a gland volume larger than 50 cc may vary compared to that of prostate carcinomas diagnosed on initial biopsy. In these cases special emphasis on the apico-dorsal peripheral and transitional zones should be considered [54, 55]. Biopsy of the SV is not recommended unless a palpable abnormality is appreciated.
It is generally recommended that 10–12 biopsies of the gland be obtained with attention to the anterior horns. Technique is important in obtaining laterally directed biopsies. It should be recalled that the tru-cut needle travels between 17 and 24 mm depending on the manufacturer’s standard. The user must appropriately guide the angle of the biopsy to maximize its position laterally on the gland, particularly when the anterior horns are approached (Fig. 11.5).


Fig. 11.5
Transrectal ultrasound image (7.5 MHz) of the prostate—transverse view. White arrow depicts the anterior horns of the prostate
Repeat Biopsy
For the patient who has had a previous negative biopsy but has a persistent PSA elevation or abnormal DRE, an additional biopsy may be considered. During repeat biopsy the standard extended-core biopsy protocol should be carried out. Additionally any sites of ultrasound abnormality and any sites of high-grade prostatic intraepithelial neoplasia (HGPIN) or ASAP should be sampled [56]. The aforementioned strategy of apico-dorsal peripheral and TZ biopsies should be included. It is well established that as successive biopsies are obtained, a decreased rate of prostate cancer detection will be observed with each additional biopsy [57]. A large series of over 1100 men undergoing biopsy as directed by PSA screening found an initial prostate cancer detection rate of 34 % [58]. This declined to 19, 8, and 7 % on biopsies 2–4, respectively. This was echoed by Djavan in the European Prostate Cancer Detection Study [59]. Thousand and fifty-one men with a PSA value of 4–10 ng/mL had a detection rate of 22 % on primary biopsy. This declined to 10, 5, and 4 % on subsequent biopsies 2, 3, and 4. The use of free and total PSA, along with prostate cancer antigen-3 (PCA3), may allow risk stratification of patients for additional prostate biopsy. Catalona et al. [60] proposed the use of the percentage of free PSA to reduce unnecessary biopsies in patients with PSA values between 4.0 and 10.0 ng/mL and a palpably benign gland. In this study Catalona suggested that patients with a free PSA of less than 25 % were significantly more likely to have a positive biopsy. PCA3 encodes a prostate-specific messenger ribonucleic acid (mRNA) that serves as the target for a novel urinary molecular assay for prostate cancer detection [61]. It has also shown promise as an aid in prostate cancer diagnosis in identifying men with a high probability of a positive (repeat) biopsy. Urine is collected after DRE, and PCA3 mRNA concentration is measured. Haese et al. [62] compared PCA3 to percent-free PSA and biopsy results in 463 men undergoing repeat biopsy. The overall positive repeat biopsy was 28 %. The probability of a positive repeat biopsy increased with rising PCA3 scores. The PCA3 score (cut point of 35) was superior to percent-free PSA (cut point of 25 %) for predicting repeat prostate biopsy outcomes.
Saturation Biopsy
Saturation biopsy has a role in maximizing prostate cancer detection rates in select patients at high risk for prostate cancer in the setting of a negative biopsy. Protocols have been described using both transrectal and transperineal approaches [63]. Various series have shown detection rates of 30–34 % [64–67]. A drawback to this procedure is the need for the biopsies to be performed under sedation in a hospital setting. However, there are circumstances that warrant more extensive gland sampling. These include (1) a persistent rise in serum PSA, with prior negative biopsies and (2) new DRE abnormalities and low volume cancers for which a surveillance approach is being considered .
The importance of saturation biopsies lies in the fact that as high as 35 % of cancers might be upgraded with the saturation biopsy technique [68, 69].
In addition, about a third of patients have exclusively anteriorly located tumors, which can best be sampled with transperineal saturation biopsies [70, 71]. The standard transrectal approach has a theoretical disadvantage due to its limited ability to obtain anterior prostatic tissue particularly at the apex. The technique described later focuses on the transperineal approach with glandular mapping.
Transrectal Ultrasound-Guided Transperineal Prostate Biopsy Using the Brachytherapy Template
Indications for transrectal ultrasound-guided/transperineal prostate biopsy (TRUS/TPB) include men with further worrisome serum PSA changes or DRE abnormalities after one or more negative standard biopsies or those considering active surveillance for prostate cancer. The TRUS/TPB includes stereotactic TPB using a standard brachytherapy template and the ultrasound device together with a brachytherapy stepper device [72].
Patients are appropriately counseled on the risks and benefits of the procedure, and surgical consent is obtained. Similar prebiopsy precautions are taken as per those outlined for the transrectal technique.
Under general anesthesia, patients are placed in the lithotomy position. Intravenous antibiotics are administered. A DRE is again performed and any lesions noted. As in the case of transrectal biopsies the prostate is scanned in gray scale and color Doppler, and any abnormalities are recorded. The transrectal probe is placed in a brachytherapy stepper device and synchronized with the ultrasound device. Prostate volumes are again obtained and recorded. The prostate is divided into 12 sections: four quadrants at the base, mid-gland and apex, respectively. Tissue cores are harvested using a biopsy gun beginning at the apical quadrants. Twenty-four core samples are targeted in both the sagittal and axial views. Specimens are placed in individual jars and reported accordingly. Patients are discharged with oral antibiotics and analgesics .
MRI/Ultrasound Fusion-Guided Biopsy for Targeted Diagnosis of Prostate Cancer
The overwhelming need in the evaluation of prostate cancer is the ability to differentiate significant cancer from indolent cancer. Routine gray scale and color Doppler imaging and conventional biopsy is unable to differentiate clinically significant cancer, thus leading to the problem of overdetection. While the conventional biopsy might be “blind” systematic biopsies, the MRI TRUS fusion biopsies truly map, track, and target suspicious lesions, thus increasing yield. Gray-scale Doppler ultrasound is able to recognize up to 80 % of suspicious lesions detected by MRI [73]. Taking systematic biopsies is responsible for the incidental detection of low-grade disease in both MRI-directed and conventional sono-directed biopsies.
This relatively newer technology utilizes MRI to initially detect “targetable lesions” in the prostate. Multiparametric MRI (mp-MRI) (using T2 weighted images, Diffusion-weighted images which uses movement of water molecules into and out of tumor cells, and dynamic contrast enhanced imaging) in conjunction with a powerful 3T magnetic field is initially used to classify “suspicious lesions” based on the Prostate Imaging Reporting and Data System-(PI-RADS) score.
The MRI is performed prior to the TRUS, and is read by a specialist uro-radiologist, who identifies a “targetable” lesion, and assigns a PI-RADS score to it. These images are stored on the software. There are currently five FDA approved fusion devices (Artemis, Eigen CA; Philips Percunav, Koelis Urostation,Hitachi/HI-RVS and Biojet). At the time of the biopsy, transrectal ultrasound is used to visualize the prostate in the usual manner, with the MRI images which highlight the tumor can be superimposed over the ultrasound in real time. This “fusion” with real-time ultrasound is done using a mechanism called digital overlay. The fusion thus results in the formation of a three-dimensional image of the prostate, enabling accurate and easy biopsy of the “targets.”
Recently, there have been claims of mp-MRI and fusion biopsies to have a good negative predictive value (NPV), thus being accurate enough to rule out indolent prostate cancer, while detecting clinically significant prostate cancer [74, 75]. It is yet to be determined whether the transperineal approach or the MRI-based approach is more accurate in determining the presence of aggressive disease in patients originally thought to be candidates for surveillance .
TRUS Biopsy After Definitive Treatment and Hormonal Ablative Therapy
External beam radiotherapy (EBRT) decreases the size of the prostate gland. Small areas of cancer which have moderate or severe radiation effects tend to appear isoechoic while large (greater than 4 mm) foci of cancer usually show little radiation effect, and these foci typically appear hypoechoic [76]. In situations that are worrisome for biochemical relapse and where the urologist and patient are seeking proof of local relapse, prostate needle biopsy is usually performed as described in the transrectal ultrasound-guided manner.
With the exception of the presence of well-distributed foreign bodies, long-term changes after brachytherapy resemble EBRT [77]. Biopsies of the gland are obtained in the same fashion as described earlier.
Whittington [77] described the effect of LHRH analogs on prostate tissue. The median decrease in prostate volume as a result of androgen deprivation was 33 %. The reduction in volume was greatest in men with the largest initial gland volume (59 %) and least in men with the smallest glands (10 %). It is rare that further biopsies will be required after hormonal ablation; however, if biopsies are required, it is very important to notify the pathologist as to the presence of hormonal ablation given the histologic changes that usually occur in these situations.
After radical prostatectomy, the presence of lesions (hyper or hypoechoic) interrupting the tapering of the bladder to the urethra, representing the anastomotic plane, is considered worrisome for recurrence [78]. One notable exception is nodules anterior to the anastomosis which may be the ligated dorsal venous complex [79]. Biopsies of the bladder neck–urethral anastomosis are possible. The target for biopsy is usually small, and care is required to accurately map the anatomy prior to biopsy. The typical area of concern is between the bladder neck and the external sphincter. The urologist is reminded to exhibit extreme caution around the sphincter .
Complications
Transrectal ultrasound-guided needle biopsy is safe for diagnosing prostate cancer. Complications do arise after a prostate needle biopsy with few major but frequent minor self-limiting complications (Table 11.2).
Table 11.2
Complication rates in TRUS biopsies
Complication | Incidence |
|---|---|
Vagal response secondary to pain/anxiety | 1.4–5.3 % [67] |
Hematuria | 71 % |
47 % resolved over 3–7 days [81] | |
Hematospermia | |
Rectal bleeding | |
Acute urinary retention | 0.4 % [112] |
Bacteremia (with enema) | 44 % [83] |
Bacteriuria (with enema) | 16 % [83] |
Vagal response, secondary to pain/anxiety, occurs in 1.4–5.3 % of patients [67]. Vagal response is generally responsive to hydration and placing the patient in the Trendelenburg position. Hematuria is quite common immediately following biopsy (71 %), with 47 % of patients having limited hematuria resolving over 3–7 days [74]. Hematospermia is observed in 9–36 % of biopsies and may persist for several months [7, 72]. Rectal bleeding is observed in 2–8 % of biopsies [7, 74]. Frequently, the bleeding is mild and can be controlled with digital pressure. In severe cases where bleeding is not controlled with conservative approaches, bleeding may be managed with rectal packing [75, 80], using a tampon or gauze, or can be addressed with endoscopic injection of vasoconstrictive agents or ligation of bleeding vessels during colonoscopy [76]. Acute urinary retention requiring catheter drainage occurs up to 0.4 % of the time [76]. Men with enlarged prostates or with severe baseline lower urinary tract symptoms are at an increased risk [81, 82]. In the preprophylaxis era, Thompson [83] demonstrated a bacteremia rate of 100 % with a bacteriuria rate of 87 %. This decreased to 44 % and 16 %, respectively, with enema alone [83]. Crawford et al. [6] administered 48 h of carbenicillin and noted bacteriuria to decrease from 36 to 9 % versus the control group. The treatment group’s incidence of fever was 17 % compared to a rate of 48 % in the control group. Antibiotic prophylaxis is now standard of care. Berger demonstrated fever (>38.5 ○C) in 0.8 % of 4303 patients receiving a 5-day course of ciprofloxacin [7]. Similarly, a study administering 1–3 days of ciprofloxacin noted a fever incidence of 0.6 % [84]. Seeding of prostate cancer in the needle tract is rare but is reported in the literature. It is seen more commonly in the form of perineal recurrences after transperineal biopsy [85, 86] but has been reported after TRUS biopsy [87]. Perineal recurrence has a poor prognosis [86], while rectal seeding has been shown to be responsive to hormonal therapy as well as EBRT [88]. Hara demonstrated increased circulating PSA mRNA in men following positive biopsy; [89] however, the risk of developing metastatic disease is thought to be low [56].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree