19 Ultrasound in the Assessment and Management of Arterial Emergencies
Ruptured Abdominal Aortic Aneurysm
An aneurysm is a localized dilatation of an artery, with an increase in diameter of greater than 50% of the normal size. Abdominal aortic aneurysms (AAAs) are most commonly encountered in the infrarenal region. In general, AAAs occur when the maximal anteroposterior diameter is greater than 3 cm. As the population ages, the incidence of AAAs is increasing. Approximately 1.5 million Americans have AAAs, and 200,000 are diagnosed each year.1 Most AAAs are asymptomatic and are detected during routine physical examinations or radiologic procedures for other problems. Elective repair is reserved for subjects with AAAs of at least 5 cm in diameter, and postoperative survival is approximately 95%. Symptomatic aneurysms may result in abdominal, flank, or back pain, distal embolization, thrombosis, or rupture. The latter complication is usually fatal if untreated; surgical repair carries a 30% to 65% survival rate. Success is highly dependent on rapid diagnosis and transport to the operating room.2
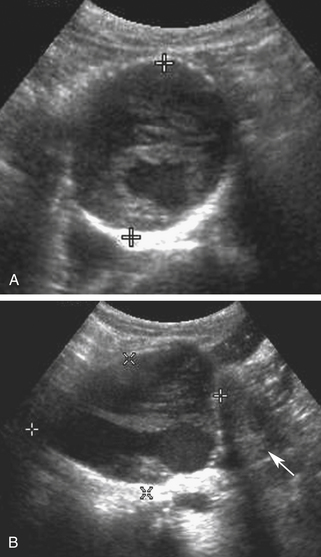
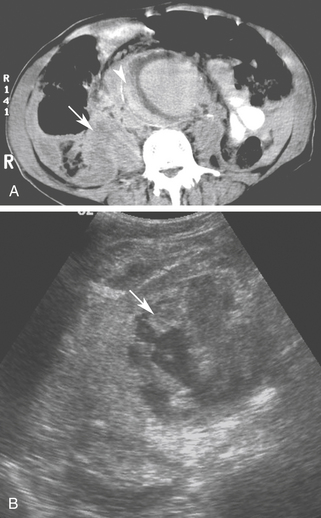
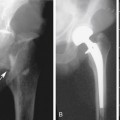
Although noncontrast computed tomography (CT) is also useful for this purpose, ultrasound is more expeditious in these potentially unstable patients. Ultrasound diagnosis of an abdominal aneurysm has a sensitivity of 98% and specificity of 95% in the setting of abdominal pain and hemodynamic instability.3 Although active extravasation is never brisk enough to demonstrate by Doppler ultrasound, the resultant hematoma provides evidence of rupture (Figure 19-1). Initially, intramural hemorrhage may be visible as an echogenic crescent within the aneurysmal wall. Following rupture, blood initially accumulates in the para-aortic space, extending toward the flanks via the pararenal space. Hemorrhage may track along the course of the iliac arteries to the extraperitoneal spaces of the pelvis (Figure 19-2). Anterior extension may transgress the posterior peritoneum, resulting in hemoperitoneum.
Nonetheless, conventional sonography has some limitations in imaging patients with a ruptured aneurysm because retroperitoneal hematoma is not always readily apparent, and the rupture itself is not directly visualized. In the appropriate clinical setting, contrast-enhanced sonography can be performed in less time than is required for contrast-enhanced CT. Findings associated with aneurysm rupture in these studies include delayed or protracted aortic lumen opacification, contrast leakage through luminal thrombus or around the aneurysm, and dependent contrast pooling around the aneurysm.4 There are no large studies evaluating the diagnostic accuracy of ultrasound in patients with suspected acute rupture of an AAA.
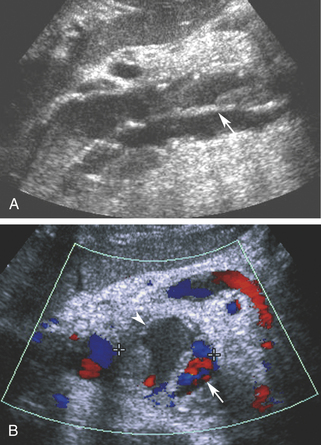
Aortic dissection may be encountered as an alternative diagnosis in patients with a clinical presentation suggesting aneurysm rupture. Although ultrasound is not a primary diagnostic modality, since it cannot reveal the full extent of dissection in the thorax, the characteristic intimal flap and altered blood flow pattern are readily visible on abdominal sonography and can lead to appropriate workup and treatment (Figure 19-3).5 In a recent study, the use of contrast-enhanced sonography increased the sensitivity for abdominal aortic dissection to 97%, from 68% for combined gray scale and duplex sonography.6 Additional details about aortic aneurysm and dissection are presented in Chapter 27.
Carotid Artery Stenosis
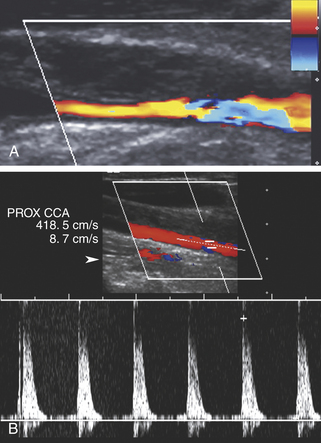
Sonographic detection and quantification of carotid stenosis in the emergency setting (i.e., progressive neurologic deficit) is similar to the nonacute setting and is described in Chapter 9. It is important to distinguish acute carotid thrombosis from stable (chronic) carotid occlusion. Whereas revascularization of a chronic occlusion is generally contraindicated, timely intervention may avoid or limit neurologic sequelae in cases of acute thrombosis.
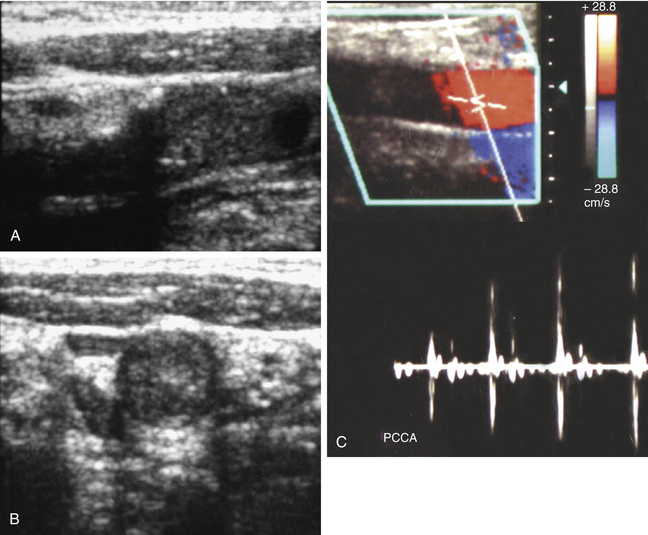
Acute thrombosis may occur as a complication of endarterectomy or stenting, or it may represent acute progression of carotid stenosis. The thrombus is usually heterogeneously echogenic (Figure 19-4). The vessel lumen is of normal caliber or slightly expanded, as opposed to chronic occlusion, which may result in luminal narrowing or obliteration.7 Pulsations may be observed in the vessel wall, which retains its normal compliance. Swirling, sludgelike flow may be observed in the carotid bulb at the interface of the thrombosed and patent lumen. Spectral waveforms assume a high-impedance, hammer-like, or to-and-fro configuration proximal to the thrombus. Internalization of the external carotid artery waveform is uncommonly seen with acute thrombosis due to insufficient time to develop collateral pathways.
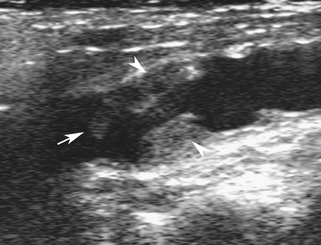
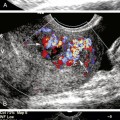
If the thrombus is nonocclusive, its free edge may oscillate back and forth in the bloodstream; the “free-floating” thrombus has a characteristic appearance at real-time examination (Figure 19-5).8 The risk for embolization is related to the extent of attachment of the base of the thrombus to the vessel wall; this can be depicted with the aid of color Doppler or B-flow imaging.9
When patients with acute carotid thrombosis undergo thrombectomy or revascularization, intraoperative ultrasound is a useful adjunctive technique. Scanning directly over the vessel in the exposed surgical field yields superb resolution of the vessel wall. It is possible to visualize small intimal flaps, ulcerative plaques, or retained thrombi, leading to immediate surgical revision, which favorably impacts patency rates.10
Carotid Artery Dissection
There are two types of carotid artery dissection. The “primary” dissection is in the cervical internal carotid resulting from hemorrhage into the wall of the carotid artery and extending distally to the carotid canal in the petrous portion of the temporal bone. The second type of carotid dissection usually extends into the common carotid artery from a type A dissection of the aortic arch.
We will be discussing the primary type of internal carotid artery dissection where the media is the most common location for hemorrhage, with extension into the subintimal or subadventitial layers. The former may result in thrombosis of the vessel; aneurysms may occur from the latter. Spontaneous dissections may be associated with type IV Ehler-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and cystic medial necrosis; however, most spontaneous dissections are idiopathic. They often happen in previously healthy individuals younger than 40 years.11 Most traumatic carotid dissections result from motor vehicle accidents in which the neck is hyperextended, compressing the carotid artery against the atlas or second cervical vertebra. Blunt trauma, penetrating injuries, and catheter injuries during arteriography also cause traumatic dissections.12
Patients with carotid dissection often complain of neck pain, headache, tinnitus, or a focal neurologic deficit. Horner syndrome, transient monocular blindness, neck swelling, and cranial nerve palsy may also result. The onset of symptoms may be hours to days after the dissection occurs.13
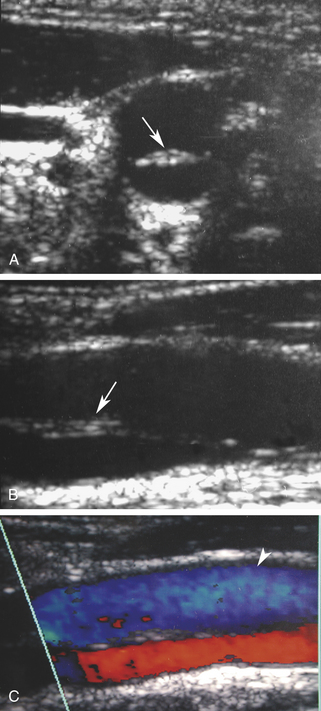
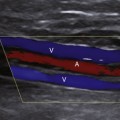
Duplex scanning of patients with suspected carotid dissection can be performed quickly in the emergency department to make this diagnosis. Typical findings with duplex scanning include a patent carotid bifurcation with tapering of the internal carotid artery, leading to a distal stenosis or occlusion (Figure 19-6). Intimal flaps or membranes may be seen (Figure 19-7), and spectral analysis reveals high-resistance waveforms with reduced flow velocity in the internal carotid artery.14 Ultrasound demonstrates a sensitivity of 70% for the diagnosis of spontaneous dissection in the cervical carotid artery, and 75% to 86% in the vertebral artery.15 Follow-up scanning can be useful to monitor vessel recanalization and to determine the duration of anticoagulation therapy.