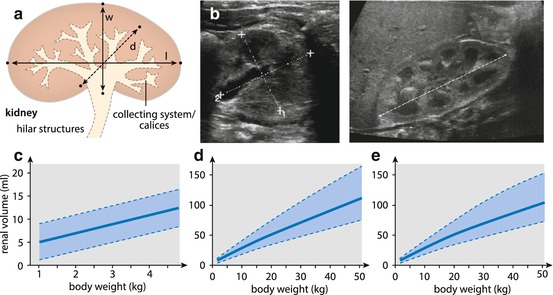
Fig. 10.1
Normal bladder US. (a) Schematic drawing with respective images demonstrating standard measurements for volume calculation. Correction factor varies with bladder shape. Additionally bladder wall thickness may be an indicator for bladder pathology (>2 mm in full, >4 mm in empty bladder), L length, W width, D depth. (b) Longitudinal (extended view technique is helpful in large-size bladders for proper measurement); (c, d) axial section – with axial measurements (callipers) for volume assessment; calculation depends on bladder shape that defines the correct correction factor: in sphaeric-ellipsoid shape factor = 0.5 (c), in a rectangular shape it is 1 (d). Note additionally thickened and trabeculated bladder wall. (e, f) Open bladder neck in axial (e) and sagittal (f) view – a potential sign for bladder instability or other functional disturbance. (g) Ureteric inflow jet – best seen on CDS: note the asymmetry in this example
Assess both kidneys from ventral, lateral and dorsal in longitudinal and cross sections:
Evaluate entire organ; document any abnormalities in two planes.
Assess: shape, size, position, contour, parenchymal echogenicity and cortico-medullary differentiation, collecting system dilatation (if enlarged, measured in axial plane) and thickening of pelvic wall and peripelvic fat/fibrotic tissue.
Calculate renal volume (L × W × H × 0.53).
Assess vascular anatomy (CDS) (e.g. accessory renal artery); follow vessels to origin or drainage (e.g. retro-aortal left renal vein). Add spectral analysis if indicated from main as well as intrarenal vessels (from upper, middle and lower segment).
aCDS is applied for peripheral vasculature (e.g. focal perfusion defects?).
NOTE: Physiologic difference in colour intensity between highly vascularised cortex and less vascularised medullae. Compare both sides in relation to perirenal structures.
Always assess perirenal space (adrenal gland).
Try to assess pelvi-ureteric junction (open? obstructed by vessel? kinking?…); follow ureter downwards, if dilated.
Assess collecting system after voiding (changes in dilatation?).
CDS: Document ureteric jet into bladder (symmetric? position of ostium?), vascular anatomy extrarenally as well as main intrarenal arteries (proper scale setting not to miss aliasing and turbulences at sites of possible stenosis or AVF…). Assess for possible twinkling (calcifications and sedimentations).
10.1.5.1 Diuretic US
Used for assessment in hydronephrotic kidneys: standardised diuretic stress induced by medication (e.g. furosemide, dose = 1 mg/kg, up to 20 mg) given orally or IV (acts faster). Repeat assessment of kidney till findings (dilatation of collecting system, Doppler flow spectra) have returned to baseline – delayed or missing normalisation of flow patterns after diuretic stress indicates significant obstruction.
10.2 Normal Findings
10.2.1 Bladder
Well-filled bladder has smooth contour and muscular wall, not >2–4 mm thick (depends less on age than on filling):
Ostium at latero-cranial end of bladder trigone as well as distal ureters often seen physiologically in well-filled bladder and good-hydrated children.
Assess bladder volume (see above and Fig. 10.1). Simple equation often used to define normal range: volume (ml) = [age (in years) + 2] × 30.
Bladder neck closed unless there’s an urge to void; after voiding bladder neck should be closed. Open bladder neck, thickened bladder wall, irregular inner contour with trabeculation and pseudo-diverticulae, high bladder tension or atypical contour may raise suspicion of neurogenic bladder/functional disturbance.
For assessing retrovesical structures (distal ureter, retrovesical space, internal genitalia, etc.), adequate manipulation of TGC adaptation necessary. Use ureteric jet for defining ostium position and assessing symmetry of urine inflow.
NOTE: Nodular wall component at bladder roof may be physiologic as remnant of urachus. Furthermore in neonates the urachus may still be depicted for a couple of weeks but without patent lumen. In neonates with still immature bladder function, some residual urine does not indicate pathology.
10.2.2 Kidney
Parenchymal appearance changes with age:
Neonatally – relatively thick parenchyma with accentuated cortico-medullary differentiation and rather echogenic cortex (may be more echogenic than adjacent liver tissue), with hypoechoic medullae. Distal medullae and papillae may exhibit echogenicities (transient physiologic phenomenon that resolves spontaneously) (Fig. 10.2). With age, cortico-medullary differentiation gets less pronounced; echogenicity of cortex decreases. Echogenicity of distal medulla becomes homogeneously hypoechoic (Fig. 10.3).
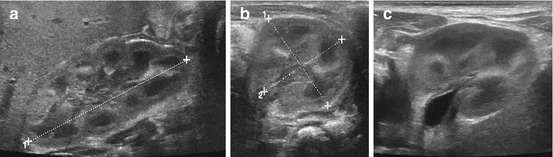
Fig. 10.2
Normal neonatal kidney. (a) Longitudinal section of right neonatal kidney with length measurement (+ ….+). Note physiologically relatively high echogenicity of cortex (similar to liver), pronounced cortico-medullary differentiation and some (transient) echogenicity in distal medulla. (b) Axial section of left kidney with measurements (callipers) – same appearance of parenchyma as in (a). (c) Axial section, neonatal left kidney: same parenchymal appearance as in (a, b) but some widening of renal pelvis (<5 mm) with some slight prominence of the pelvic wall (<1 mm) – this may be normal, but may rectify a follow-up study
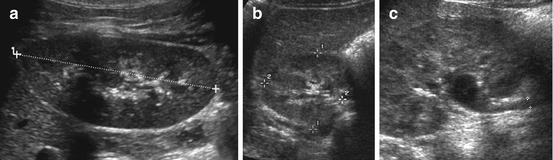
Fig. 10.3
Normal kidney in childhood. (a) Longitudinal kidney scan (from dorsal) with length measurement. (b) Axial scan of right kidney through liver with measurement of diameter. (c) Depictable extrarenal pelvis and proximal ureter (+ +), may be normal in a well-hydrated child
Sometimes papilla and pelvic wall become more echogenic with some slight echogenicity around central structures due to peripelvic fat and fibrosis.
Calices and renal pelvis may be visible, particularly with modern high-resolution transducers, without indicating urinary transport alteration (Fig. 10.4).
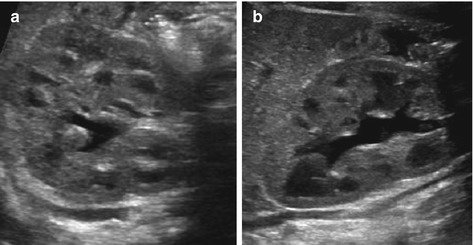
Fig. 10.4
(Normal) collecting system in neonates and infants. (a, b) Normal neonatal kidney, despite (physiologically) prominent cortico-medullary differentiation and some dilatation of the collecting system – but with normal configuration of fornices and papillae (HNII)
Assess fornices and papilla to demonstrate normal shape; assess renal pelvis wall (should not be thickened). In the hilum, the renal vein and artery are to be recognised and can be differentiated by following these structures to their vascular origin, whereas the pelvis curses downwards towards ureteropelvic junction.
Renal size: standardised measurements and volume calculation; compare with age-/weight-adapted growth charts (Table 10.1). Always compare to other side (calculate relative renal volume: volume right + left = 100 %). If different/one kidney enlarged or smaller, always meticulously try to assess potential reasons.
Table 10.1
Renal size – normal values (according to Weitzel)
CDS: After finding vessels, identify them by flow direction; perform spectral analysis from duplex trace. Slightly higher arterial RI (0.75–0.80) physiologic in neonates, then quickly maturing to normal adult values (RI ~ 0.67 ± 0.03) (Fig. 10.5). Also observe shape, particularly of systolic inflow – flattened and slow acceleration of systolic upstroke typical for renal artery stenosis.
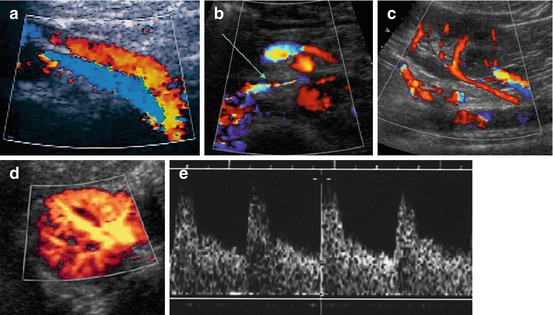

Fig. 10.5
Normal aCDS of kidney. (a) CDS of main renal vessels – median ventral access: left renal vein (red) running in front of renal artery (encoded in blue) and passing anteriorly to abdominal aorta. (b) Further normal course of left renal vein (arrow) between abdominal aorta and superior mesenteric artery till entering inferior vena cava (IVC); due to some narrowing increased flow velocity and some flow turbulence causing aliasing on CDS. (c) Accessory renal arteries, which can be a normal variant, but may also be associated with urinary obstruction due to vascular impairment of proximal ureter/pelvi-ureteric junction. (d) Normal aCDS of a healthy kidney – axial section. (e) Normal duplex trace of main/segmental renal artery
10.2.2.1 Normal Variants
Number of variations that do not necessarily indicate pathology: parenchymal bridge/hypertrophic column of Bertin in duplex kidney, rotation/position anomalies, caliectasia, extrarenal pelvis, persisting renunculae, other contour alterations (e.g. “splenic bump”), variations in vessels such as accessory renal arteries/veins and retroaortic left renal vein (Fig. 10.6).
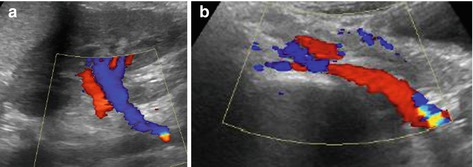

Fig. 10.6
Retro-aortal left renal vein. (a) Abnormal course of left renal vein accessed through kidney from a longitudinal (coronal) flank view: blue-coded vein courses distally instead of running cranially parallel to renal artery. Note some aliasing at the area the vein reaches abdominal aorta. (b) Ventral median axial view: left renal vein (encoded red) passes behind abdominal aorta with tapering due to some compression between aorta and vertebral body
Duplex Kidney
US Findings
Central parenchymal bridge, sometimes disproportionate upper and lower collecting systems, potentially associated dysplasia with lack of cortico-medullary differentiation and increased echogenicity commonly of upper pole moiety parenchyma (Fig. 10.7):
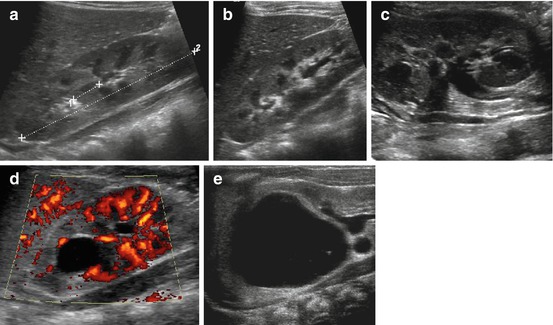

Fig. 10.7
Duplex kidney. (a) Duplex kidney (+…+2), longitudinal flank view: no distension of renal collecting system, only parenchymal bridge in middle of kidney (1+ …+) interrupting central pelvis echoes indicates duplication. (b) Duplex kidney, longitudinal flank view: disproportional dilatation of lower collecting system. (c) Duplex kidney, longitudinal flank view tilted medially: two separate ureters of upper and lower moiety seen, with different grade of distension of respective collecting system. (d) Duplex kidney, longitudinal flank view: significant dilatation of upper moiety with unstructured, narrow, echogenic parenchyma; lower moiety exhibits normal parenchymal structure: NOTE: significantly reduced vascularity on aCDS in dysplastic upper moiety. (e) Huge, cystiform dilatation of upper moiety of duplex kidney with rim-like residual dysplastic parenchyma, only slight dilatation of lower system – not to be mistaken for a renal cyst
Obstruction most commonly in upper system, rarely involves lower pole system.
Meyer–Weigert rule – ureter of lower system drains latero-cranially into bladder with risk of VUR, whereas ureter of upper pole system usually drains distal-medially, may even insert ectopically, may be associated with ureterocele and often is obstructive.
Lower system usually reflexes (reflux nephropathy? secondary obstruction/kinking?).
Always try to assess whether there is one common extrarenal pelvis or two ureters leaving kidney; also try to visualise precise ostial anatomy (1 or 2 ostia? 2 jets? etc.).
Ectopic Kidneys
US Findings
May be anywhere in the retroperitoneum, may be small and dysplastic, sometimes only cystic remnants are seen. Even intrathoracic position possible – then close to diaphragm and paramedian dorsally (Fig. 10.8).
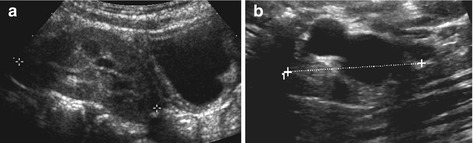

Fig. 10.8
Ectopic kidney. (a) Pelvic ectopic kidney (+ +) visualised behind and above well-filled urinary bladder, sitting in front of spine. (b) Cystic dysplastic ectopic kidney (+ +) sitting in front of psoas muscle distally to its normal position, only seen by meticulous search using graded compression; some residual parenchyma seen – ureter was draining ectopically into vagina causing constant dribbling
NOTE: Small ectopic kidneys may drain ectopically and can still have function – this may cause symptoms such as urinary dribbling (when inserting into vagina or distal to sphincter). If both kidneys not seen in normal position; meticulous search of entire abdomen mandatory.
Renal Agenesis
Only compatible with extrauterine life only if unilateral – single (hypertrophic) kidney on other side. Often constitutes end point of disturbed fetal development, e.g. dysplastic or cystic kidney regressed and cannot be seen any longer. In these children high probability of ipsilateral genital malformation – should be explicitly searched for.
NOTE: Bilateral renal agenesis fatal, usually diagnosed prenatally, leads to severe hypoplasia of lung.
Fusion Anomalies and Other Rare Findings
Horseshoe kidney – most common anomaly: lower poles attached at midline, form parenchymal bridge in front of aorta (Fig. 10.9); associated with impairment of urinary drainage, higher risk for being injured in abdominal trauma.
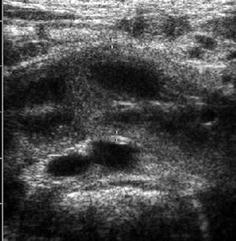

Fig. 10.9
Horseshoe kidney. Parenchymal bridge (callipers) of a horseshoe kidney depicted in front of aorta, IVC and vertebral body connecting right and left moiety; mesenteric vessels seen in front of renal parenchyma in this axial abdominal midline section
Cross–fused dystopia, triple kidney and other rare variations – not addressed in detail.
Bladder anomalies – such as duplex bladder rare; bladder exstrophy not target of US investigations (only for assessing kidneys).
10.3 Pathology of the Kidney
10.3.1 Congenital Conditions
10.3.1.1 Dysplasia/Hypoplasia
Definition
Structural alteration of renal tissue with more or less impaired function; may be focal or diffuse, unilateral or bilateral; often associated with severe obstructive uropathy and/or VUR; congenital/inherited or syndromic.
US Findings
Commonly increased parenchymal echogenicity with reduced cortico-medullary differentiation, potentially with cysts (Fig. 10.10) – see also cystic kidney disease (below) May be smaller.
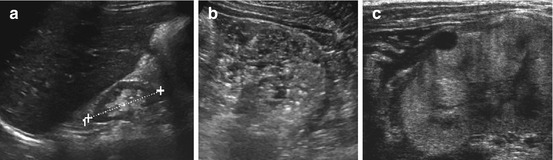

Fig. 10.10
Hypodysplastic and cystic kidney disease. (a) Small, relatively normal looking kidney (+ +) in a child with end-stage renal failure due to bilateral renal hypodysplasia. (b) Dysplastic kidney with completely disrupted parenchymal structure (similar as in ARPKD (“pepper and salt” appearence)). (c) Echogenic widened cortical parenchyma with cortical cyst in another cystic-dysplastic malfunctioning neonatal kidney in systemic-syndromatous disease
CDS: Potential cortical vascular rarefaction with increased resistance (RI ↑), depending on functional impairment; also pseudonormal flow patterns with reduced systolic velocity.
10.3.1.2 Cystic Renal Disease
Definition
Often inherited, genetic, congenital condition – even if cysts only manifest later in life.
However, secondary and acquired cystic disease exists, as well as cystic tumours.
US Findings
Usually anechoic structures with dorsal amplification (Fig. 10.11). Depending on size, content and border, classified into various degrees of complexity (adapted from the Bosniak CT Classification, though without having enhacement pattern available on US: from grade I = uncomplicated simple cyst to grade IV being a severely complicated, highly suspicious cyst). Classification of cysts – see Table 10.2:
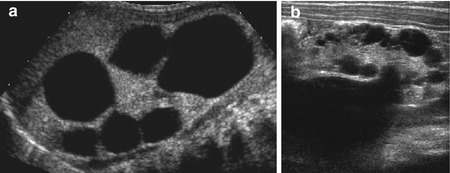
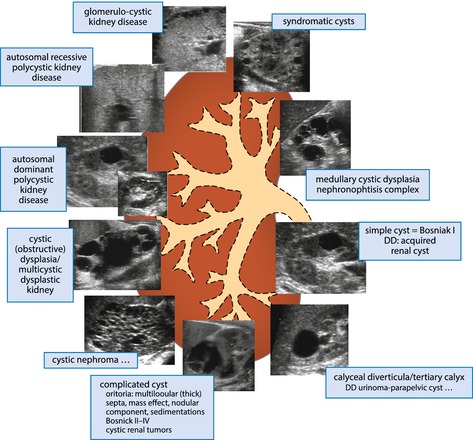

Fig. 10.11
Cystic kidneys: dysplastic cyst, multicystic dysplastic kidney (MCDK). (a) Typical MCDK with large, not connected, peripheral cysts; some central echogenic dysplastic residual parenchyma. (b) Severe fetal obstructive uropathy with multiple cortical cysts and dysplastic parenchyma in a neonate
Table 10.2
Schematic drawing for DDx of renal cystic conditions

Simple cyst: (isolated/single) uncomplicated cyst (no content/completely anechoic, smooth margin, thin wall, no solid component, etc.), localised, with or without growth. Rarely seen in neonates and infants.
Multiple cysts: often associated with parenchymal dysplasia or genetic/inherited cystic kidney disease.
Complicated cyst(s): as anywhere else – internal echoes and sedimentation, septae, thick wall, irregular border, solid/nodular components, conglomerates of cysts, large, irregular shape; vascularised compartments best seen by aCDS . These need follow-up/diagnostic workup, sometimes additional imaging.
Inherited/Congenital Cystic Disease
Often part of ciliopathy complex (autosomal dominant and recessive cystic kidney disease – ARPKD and ADPKD; glomerulocystic and medullary cystic kidney disease – GCKD and MCKD; and nephronophthisis/tuberous sclerosis complex) (Table 10.2):
ARPKD: enlarged hyperechoic kidneys bilaterally, with disrupted echotexture and potential regional echogenicities (“salt and pepper appearance”) due to microcysts (too small to be resolved by US), with or without some dilatation of collecting system. Often associated with liver fibrosis (maybe initial manifestation of disease) (Fig. 10.12).
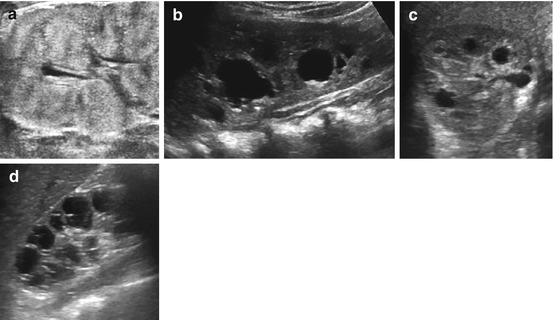
Fig. 10.12
Hereditary/genetic cystic renal disease: ARPKD, ADPKD, nephronophthisis. (a) ARPKD – bilaterally enlarged kidney, in neonates microcysts often not visible by US, some patchy “pepper and salt” appearances of hyperechoic parenchyma. (b) ADPKD – bilateral (groups of) single cysts of different size (child with known familiar cystic kidney disease). (c) Syndromatous cystic renal disease – unspecific cysts in both kidneys in a neonate. (d) Nephronophthisis – girl with end-stage renal failure due to nephronophthisis. Observe the typical parenchymal, radial-grouped cysts seen in late stage of disease
ADPKD: larger parenchymal cysts, can be conglomerated or isolated, single or multiple (associated with cysts in other parenchymal organs, vascular problems such as aneurysms); need comprehensive follow-up including all respective changes (Fig. 10.12).
Syndromic cystic kidney disease: many syndromes exhibit renal cysts. Some also carry increased risk for nephroblastomatosis/malignant degeneration.
Non-genetic congenital cystic disease: often associated with urinary tract obstruction or high-grade VUR (see dysplasia), rarely familial.
Multicystic dysplastic kidney (MCDK): most common entity (see Fig. 10.11). Said to originate from severe early upper tract obstruction causing severe cystic dysplasia. Caused by impaired connection between ureteric bud and renal blastema. Classically defined by multiple large cysts with potentially some echogenic undifferentiated central parenchyma (may even exhibit residual vascularisation). Continuous transition to severe obstructive uropathy with dysplastic cysts of parenchyma – thus some residual central collecting system or residual ureter may be visible. Commonly tend to shrink/vanish spontaneously; however, some may grow, get infected, cause hypertension or undergo tumourous transformation (particularly those with vascularised residual parenchyma). US monitoring recommended.
NOTE: Often associated with ipsilateral genital malformations, similarly to solitary kidneys, where contralateral kidney may have involuted during embryology secondary to similar phenomena. Cystic renal buds may be found in ectopic position with some residual or absent function. Thus always carefully assess entire abdomen and genital tract as well as contralateral kidney, which will develop compensatory hypertrophy.
Acquired Cystic Kidney Disease
Definition
Number of etiologies occur and exhibit different features: posttraumatic cysts, postoperative cysts, acquired cystic kidney disease in renal failure and after transplantation.
NOTE: Simple renal cyst much rarer in childhood than in adults – every cyst detected in an infant and young child requires detailed assessment + follow-up.
DDx
Caliceal diverticulum, tertiary calix, cystic remnants of abscess/infection/trauma, urinoma, and cystic tumours (Fig. 10.13).
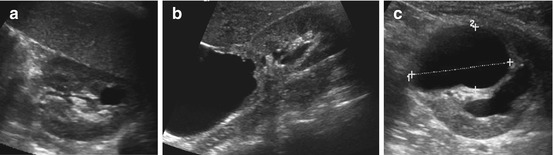

Fig. 10.13
Acquired cystic kidney disease and DD. (a) Postinfectious cyst: somewhat irregular cyst at previous site of an abscess. (b) Huge cystic mass connected with upper calyx and showing contrast extravasation on dynamic MRU, consistent with a urinoma or a huge caliceal diverticulum. (c) After heminephrectomy a growing liquid formation (1+ ….+) observed at resection site – consistent with a postoperative urinoma
Role of US
Ideal method for initial investigation/follow-up.
NOTE: Number of cysts and appearance of parenchyma do not necessarily correlate with renal function.
Additional Imaging
Depending on underlying entity – sometimes no additional imaging, sometimes assessment for VUR (VCUG/ce-VUS), renal function (scintigraphy/MR urography) or ectopic renal remnants (scintigraphy/MR).
Rarely – if unclear or suspicious for malignancy – DDx by MR (CT if MR unavailable).
10.3.1.3 Alteration of Urinary Drainage
Various underlying entities that cause pathologically altered urine flow/drainage. Often manifests fetally by “hydronephrosis” (HN).
Obstructive and refluxive entities need to be differentiated without specfic features.
Consider alternate dysplastic dilated system/ureter without impairment of urinary drainage.
Hydronephrosis (HN)
Definition
Term originally used to describe any dilatation of collecting system.
Today, due to improved resolution of US equipment, normal distension of pelicaliceal system visualised even fetally – thus HN does not definitely indicate pathology.
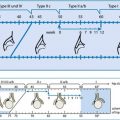
Standardised HN grading established, adapted from Society of Fetal Urology (SFU) Classification and Hoffman’s paediatric US HN grading system (HN 0° – IV°, see Table 10.3). Definition relies not on millimetre of pelvic width but visibility and configuration of collecting system and thinning of parenchyma. Grading independent of aetiology.
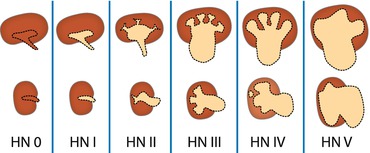
Table 10.3
HN grading in neonates and infants – according to ESPR and ESUR recommendation

Additional US signs that may indicate pathology and prompt further workup (“extended criteria”): thickening of ureteral/pelvic wall (>1 mm, nonspecific – seen in infection, oedema, obstruction, VUR), renal parenchymal pathology (e.g. cysts, altered cortico-medullary differentiation, and altered echogenicity), renal size alterations, dilatation of ureter, and bladder pathology.
Ureteropelvic Junction Obstruction (UPJO)
Definition
Narrowing/stenosis of ureteropelvic junction causing impairment/obstruction of urinary drainage. Aetiology usually congenital, may be acquired. Commonly associated with significant prenatal HN (≥II°).
NOTE: Dilatation best assessed after end of first postnatal week – as physiological renal immaturity prevents filling of collapsed dilated system during first days of life. Standardised hydration essential for proper recognition and grading, particularly for follow-up investigations.
US Findings
Dilatation of pelvi-caliceal system (HN grade III°–V°) – lower grades usually associated with non-obstructive UPJA or lower grade VUR without thinning of parenchyma and preserved cortico-medullary differentiation. Ureropelvic junction narrowed/not depictable; proximal ureter very narrow:
Pelvic ectasia: only pelvis dilated, calices visualised, but normal configuration, sometimes associated with non-obstructed ureteropelvic junction anomaly (UPJA).
HN IV° (and V°) usually indicates high-grade UPJO (Fig. 10.14).
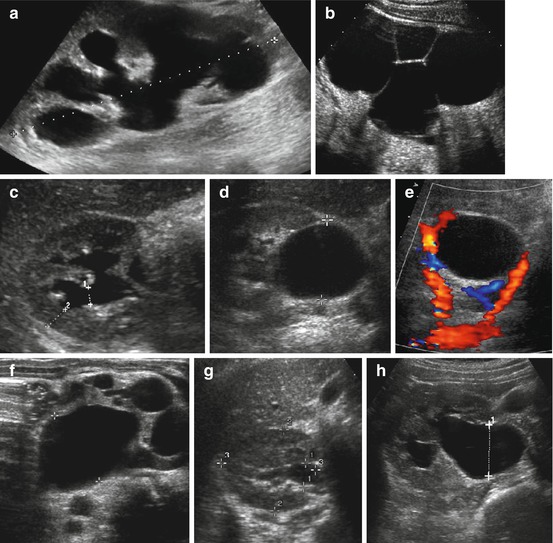
Fig. 10.14
US in UPJO (including CDS). (a) Typical appearance of high-grade “hydronephrosis” (grade IV°) with significantly dilated collecting system, thinned parenchyma and renal enlargement (+….+) in UPJO. (b) Cyst-like appearance of grossly enlarged collecting system (HN V) in UPJO; peripheral rim-like parenchyma hardly visible but enables differentiation against MCDK (i.e. central parenchyma, no connections between cystic structures). (c) Axial section of mild UPJO with only little distension of calices and caliceal neck (1+…+) and preserved parenchyma (2+….+) with persisting cortico-medullary differentiation (HN grade III°). (d) Axial section: dilatation of extrarenal pelvis (+ +) but practically no dilatation of intrarenal system. (e) Same patient as in (d) CDS reveals an additional renal artery crossing the pelvi-ureteric junction, possibly causing (intermittent) partial obstruction with dilatation of renal pelvis. (f) Gross dilatation of collecting system and pelvis in severe, fetally decompensated UPJO. Echogenic, unstructured and narrow parenchyma full with multiple cysts of different sizes (“obstructive dysplasia”). (g, h) Importance of hydration: kidney scanned in non-hydrated (g) and well-hydrated (h) state, the latter after furosemide-induced diuretic stress. Note significant change in dilatation of renal pelvis (1+ +) and collecting system impressively demonstrating importance of proper patient preparation for US studies
Signs for (chronically) decompensated obstruction: severe thinning of parenchyma, decreased parenchymal echogenicity, lack of cortico-medullary differentiation, and delayed or missing normalisation under diuretic stress by furosemide (diuretic urosonography). Potentially dysplastic cysts.
NOTE: Dilatation does not equal obstruction – US cannot diagnose obstruction. Even severe obstruction may show only minor distension under insufficient hydration/decreased function or with intermittent as well as per acute obstruction (e.g. urolithiasis). Diagnosis of severe/decompensated obstruction (which needs treatment to prevent deterioration of renal function/growth potential) relays on functional imaging.
CDS
Look for accessory/additional renal vessels that may impair ureteropelvic junction (Fig. 10.14):
In chronic and non-obstructive dilatation RI symmetric.
In acute obstruction asymmetric elevation of RI in affected kidney.
Also assess potential rarefaction of peripheral vascularity (sign of chronic decompensation with already reduced renal function/scarring), best visualised on aCDS.
Postoperative transient thickening of pelvic wall, pelvis often smaller (as reduced by surgery) and dilatation of calices often persists for long time – only normalises over years, potentially focal perfusion impairment/scar (e.g. at site of perioperative drain).
NOTE: For assessing split renal volume, the dilated collecting system has to be subtracted – best using 3DUS (allows segmentation of collecting system that can be deducted from overall renal volume, thus allowing exact parenchymal volume calculation – see Figs. 1.30 and 1.31). Can then be compared to non-obstructed contralateral side (= split/relative renal size).
Additional Investigations
MAG3 scintigraphy: gold standard for assessing renal function/urinary drainage.
IVU: outdated – replaced by MRU, indicated in complex anatomy, particularly preoperatively
In some situations – particularly postoperatively – modified focused IVU helpful by assessing anatomy/obstruction (only need few well-timed focused images).
Dynamic diuretic MRU: will in future allow for additional functional assessment.
No indication for CT, even accessory renal artery seen by US/CDS and early angiographic phase of MRU.
VCUG or ce-VUS: VUR assessment – particularly if indirect signs seen on US.
Postoperatively some perform fluoroscopic assessment of drainage before removing drain.
Uretero-Vesical Junction Obstruction (UVJO)/Obstructive Megaureter (POM/MU)
Definition
Aetiology: stenosis at UVJ or regional dysplasia of ureter with lack of peristalsis, causing impaired urinary drainage. Potentially associated with anatomic changes at ostium (low insertion, ureterocele, ectopic ureteric insertion, duplex systems, etc.).
US Findings
Dilatation of ureter, more or less thickening of wall and varying impairment of peristalsis (can be documented by video clips/M-mode) (Fig. 10.15):
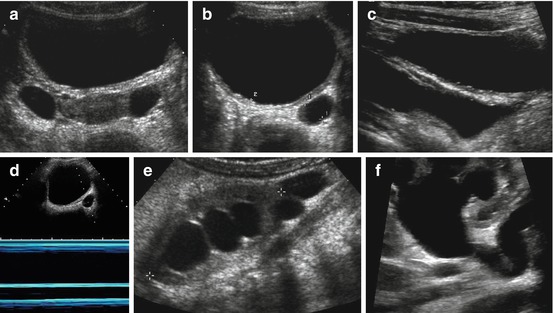

Fig. 10.15
POM/MU – M-mode. (a) Two cystiform structures depicted behind well-filled bladder lateral to uterus (proper TGC adaptation essential – automatic image optimisation programmes will not always work for this): to differentiate ovarian cysts from (bilateral) megaureter, longitudinal paramedian section is necessary. (b, c) Dilated ureter (+ +) behind well-filled urinary bladder in axial (b) and longitudinal (c) section; the latter nicely exhibiting short narrow distal/transmural section (obstructive megaureter). (d) M-mode documents lack of peristalsis in dysplastic widened ureteral segment (neonate with primary megaureter). (d) Significantly dilated renal collecting system in child with megaureter. Note cystiform fluid-filled structure below lower pole of kidney (+ +) representing loop of tortuous megaureter. (e) Pelvi-ureteric junction has kink-like anatomy explaining intermittent secondary upper obstruction in addition to megaureter
Hyperperistalsis indicates stenosis.
Lack of peristalsis hints at decompensation or dysplastic-atonic segment.
In complications (e.g. infection): echoes in ureter.
NOTE: Dilatation of ureter does not necessarily correlate with dilatation of renal collecting system – associated kink/relative UPJO will increase intrarenal distension (HN II°–V°).
CDS
No specific findings. Assessment of ureteric inflow jet may be helpful but potentially misleading. Improves depiction of ostium – thus identification of atypical insertion.
Additional Investigations
IVU replaced by (diuretic contrast-enhanced) MRU; for anatomic display T2-MRU.
VCUG/ce-VUS for differentiation of dilating VUR.
MAG3 scintigraphy – used for drainage assessment, split renal size and function and assessment of ureteral peristalsis (or dynamic MRU).
Posterior Urethral Valve (PUV)
Definition
Most common in baby boys. Has a number of forms. Severe distal obstruction often associated with upper tract pathology – high probability of congenital renal dysplasia and chronic renal failure.
US Findings
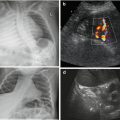
Thickened bladder wall, trabeculation, (pseudo-)diverticula, enlarged capacity, typical valve-like configuration of bladder neck (particularly well seen by perineal US during voiding attempts) (Fig. 10.16).
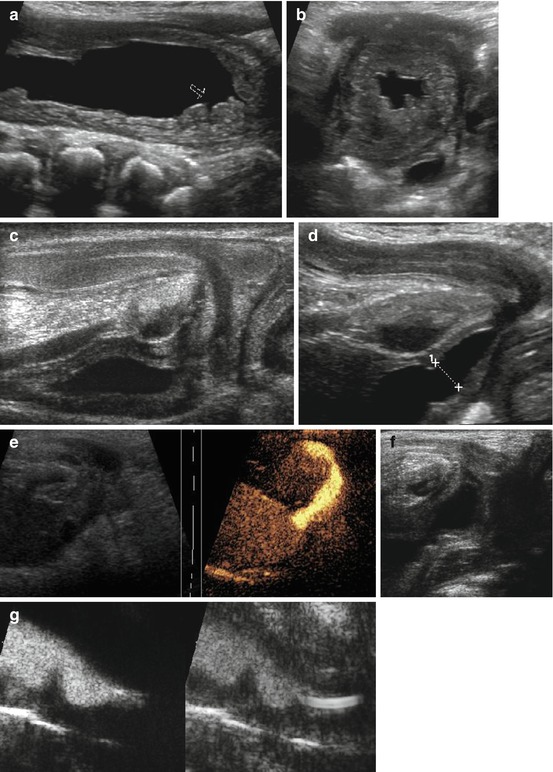
Fig. 10.16
US in posterior urethral valve (PUV). (a) Longitudinal section of large bladder with impressive wall thickening and obstruction of ureteral ostium (arrow) in baby with PUV. (b) Cross section through wall-thickened urinary bladder (after drainage via catheter) shows dilated ureter behind bladder and trabeculation. (c) Normal perineal US in baby boy (no voiding). (d) Perineal US, neonate: open bladder neck and proximal urethra to pelvic floor when trying to void – not to be confused with PUV (as on VCUG). (e) Normal male urethra during voiding on ce-US. (f) Typical valve configuration of PUV on perineal US during voiding. (g) ce-VUS: valve-like urethra configuration nicely demonstrated during voiding; catheter only seen in basic image, contrast agent better visualised in left, dedicated contrast-specific image
Secondary high-grade VUR or obstruction by thickened bladder wall, associated with more or less renal dysplasia, dilation of collecting system, potentially pop-off urinoma (Fig. 10.17) – the latter will prevent kidney from further damage.
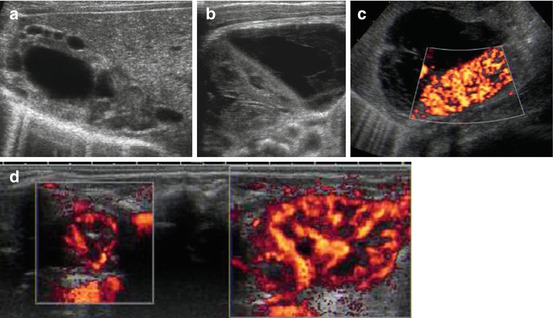
Fig. 10.17
Pop-off urinoma in PUV with renal dysplasia. (a) Dysplastic kidney in neonate with PUV, dilated pelvis and dysplastic cysts. (b) Complex fluid formation adjacent to relatively normal looking kidney in PUV, consistent with caliceal rupture and pop-off urinoma which seems to have a protective effect reducing renal damage. (c) Same neonate as in (b): good vascularisation of renal parenchyma, large urinoma in front of kidney. (d) Dual/split image technique, dorsal axial scan through both kidneys including aCDS: obviously significant difference in renal size and perfusion, left kidney severely damaged (same can also be seen in other patients with high-grade VUR without PUV)
CDS
Demonstrates renal perfusion; helps assessment of severely dysfunctional kidneys.
ce-VUS may show valve (perineal approach during voiding); VUR may show pop-off urinoma.
DDx
Prune belly syndrome (hypoplastic abdominal wall, cryptorchidism, hypoplastic prostate, hypoplasia of posterior urethra, commonly associated with complex urinary tract anomalies/dilated ureters with less-dilated intrarenal collecting system and dysplastic parenchyma).
Neurogenic bladder.
High-grade VUR.
Additional Investigations
Initial confirmation by VCUG recommended.
Early urinary drainage either by bladder relief or nephrostomy.
Renal function assessed by DMSA scintigraphy (best results after approx. third month of life – as it only works with sufficient renal function after renal immaturity has ceased).
Rarely MRU (in additional complex pathology).
No indication for IVU.
Vesico-Ureteric Reflux (VUR)
Definition
Insufficient urinary ostia (primary/secondary); reflux of urine from bladder to ureter/renal collecting system – with more or less dilatation of ureter and/or caliceal system. Grading I°–V° according to international classification.
US Findings
Indirect signs in bladder: bladder wall thickening, trabeculation, lateralisation of ostium and gaping ostium. Varying dilatation of ureter that may exhibit wall thickening. Post-void increase of dilatation of collecting system and pelvic urothelial thickening (Fig. 10.18).
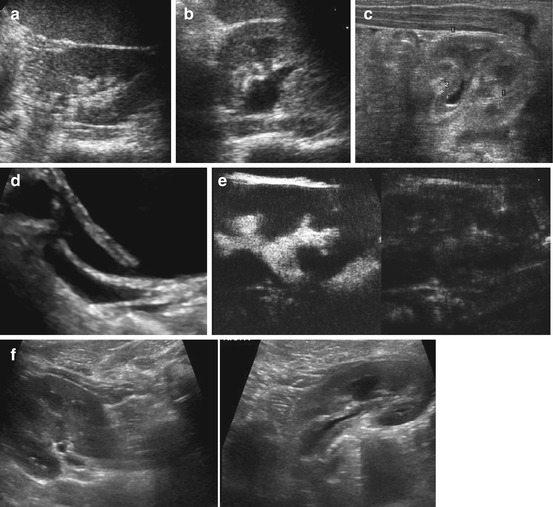

Fig. 10.18
Vesico-ureteric reflux. (a) Left kidney, axial view before voiding: no distension of collecting system/renal pelvis. (b) Same girl, same section after voiding: significant widening of the renal pelvis indicating dilating VUR. (c) Urothelial sign: thickened wall of non-distended renal pelvis – a nonspecific sign for VUR, involvement in UTI, obstruction, congestions, etc. (d) Duplex ureter – the upper and more lateralised ostium (draining lower moiety of respective kidney) is gapping, indicative of VUR. (e) ce-VUS shows contrast reflux into proximal ureter and renal collecting system. (f) Comparison of two kidneys in same patient showing different size and pelvic wall thickening associated with different degree of VUR
CDS
Ureteric inflow jet may be atypical, asymmetric and originate from lateralised ostium.
Sometimes, particularly with particles in urine, VUR can be directly visualised by reversed colour flow signals in ureter.
NOTE: These have to originate from within bladder through ostium – as otherwise reflected retrograde flow from closed ostium may mimic VUR.
ce-VUS (see also Chap. 2): reflux of US contrast agent into ureter/pelvi-caliceal system – with more or less distension, depending on VUR grade. Grading of VUR achievable by ce-VUS (correlates with standard VUR grading on VCUG – see Table 1.3, Figs. 1.24, 2.7, 2.8, and 10.18).
NOTE: Always assess renal parenchyma for signs of dysplasia/scars after infections.
Connatal dysplasia in severe VUR (common in baby boys, PUV) called “congenital reflux nephropathy”.
Postnatally VUR itself does not cause renal damage, only in conjunction with recurrent upper UTI.
Additional Investigations
VCUG – particularly in boys, preoperatively, for detailed analysis not only of urethra (also ureter, potential diverticula …). Rarely bladder function studies are performed.
DMSA scintigraphy for assessing renal parenchyma damage (might be replaced by MR in future).
IVU outdated.
Secondary Obstruction
Definition
Number of causes such as urolithiasis or compression (by tumours, retroperitoneal fibrosis, etc.).
US Findings
Acute obstruction usually does usually not exhibit significant dilatation – unless there is preexisting chronic drainage impairment/other forms of dilatation.
Increased echogenicity of parenchyma and swollen and enlarged kidney.
Potentially perirenal oedema/stranding.
Concretions/stones (urolithiasis): usually echogenic structure with dorsal shadowing, distended collecting system or ureter narrows after level of obstruction.
CDS
In acute severe obstruction, there is diminished peripheral vasculature (a)CDS. Asymmetrically elevated RI in acutely obstructed kidney.
In total obstruction, lack of ureteric inflow jet from affected ureter – otherwise asymmetric (even ipsilateral dominant) ureteric jet (e.g., from haematuria).
Twinkling sign from stone (Fig. 10.19, see Fig. 1.18).
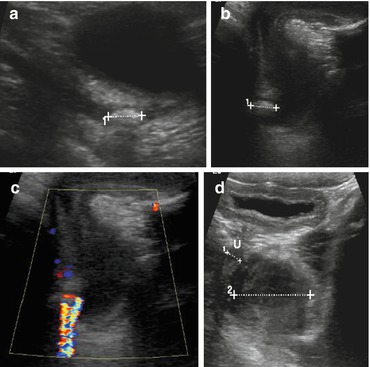

Fig. 10.19
Twinkling sign/ureteric stone. (a) Distal ureteric stone (1+ …+) with some dilatation of ureter and only little shadowing, only depictable with sufficiently filled urinary bladder. (b) Urethral stone (1+…. +) depicted by perineal US. (c) Twinkling sign caused by urethral stone (same patient as b). (d) Pelvic floor rhabdomyosarcoma, visualised through bladder (2+…+) compresses + displaces urethra (U, 1+…+), causing urethral obstruction
Role of US
Perfect initial diagnostic tool:
In children, stones can be visualised in nearly all parts of urinary tract, particularly in kidney, at pelvi-ureteric junction, in distal ureter – provided sufficiently filled bladder allows access. Even urethra (by perineal US) (Fig. 10.19d).
NOTE: Following dilated ureter downwards from renal pelvis will often allow depiction of obstructing stone even in mid/lower ureter, e.g. at pelvic vessel crossing.
Furthermore initial US allows tailoring of further examinations. Adjacent compressing structures can usually be visualised, too; potential for US-guided intervention.
Additional Investigations
Kidney-ureter-bladder film (KUB).
Focused IVU or stone CT (particularly in complex and equivocal situation where it is increasingly preferred):
Not indicated with same frequency as in adults – due to radiation burden.
Role of MR is yet undefined.
See also ESPR/ESUR recommendations – Pediatr Radiol (2010) 40:1315.
10.3.2 Inflammatory Renal Parenchymal Conditions
Role of US
Perfect initial diagnostic imaging method and for follow-up (and US-guided biopsy for DDx).
Additional Imaging
DMSA scintigraphy in acute or chronic setting (for scars, wait 4–6 months after infection):
Complicated infections or DDx of pseudotumours may require MR (or CT, if MR is not available).
IVU usually not indicated in children (rare exceptions, e.g. unless there is a suspected obstructing stone).
Additional nephro-urologic work-up of potentially underlying/associated condition recommended.
Imaging algorithm for paediatric UTI: see ESPR/ESUR recommendation Pediatr Radiol (2008) 38:138.
10.3.2.1 Pyelitis
Definition
In children isolated pyelitis is rare – potentially associated with VUR. Commonly associated with interstitial bacterial nephritis.
US Findings
Thickened echogenic renal pelvic wall and echoes within collecting system, which tends to be enlarged, hypotonic and wide (Fig. 10.20).
Secondary stone formation or fungus may be present; fungus usually similar to a stone, often larger and somewhat polygonal shaped; dorsal shadowing less than with a typical concretion, may cause secondary obstruction.
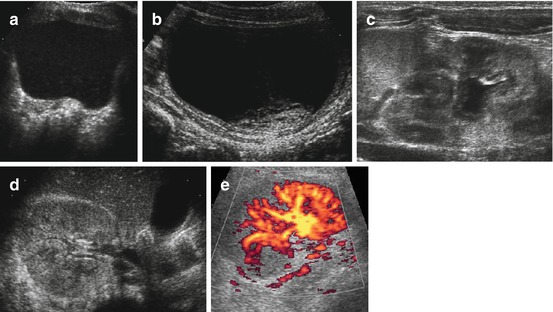
Fig. 10.20
US in urinary tract infection (UTI). (a) Echogenic content floating throughout bladder in UTI. (b) Echogenic debris (clot and fibrin) sitting at bladder base in haemorrhagic cystitis. (c) Enlarged kidney, with swollen urothelium of lax and slightly distended renal pelvis in an infant with upper UTI (acute pyelonephritis). (d) Enlarged kidney with disrupted cortico-medullary differentiation and increased echogenicity in upper UTI with diffuse renal involvement. (e) aCDS depicts multi-segmental perfusion defects in same child as (d); these local manifestations were less depictable on gray scale US
10.3.2.2 Acute Pyelonephritis (aPN)/Interstitial Nephritis
Definition
Haematogenous or ascending infection, bacterial or viral, sometimes atypical (tuberculosis).
US/CDS Findings
Focal/diffusely altered parenchymal echogenicity – commonly increased, swollen kidney, in focal infection even pseudotumours, regional swelling (“lobar nephronia”), peripelvic increased/broadened echogenicity and perirenal oedema, often associated with findings as in pyelitis.
Regionally/segmentally decreased vascularity – particularly well seen on aCDS, diffuse asymmetrically reduced vascularity on power Doppler in diffuse infection (Fig. 10.20).
10.3.2.3 Necrosis and Abscess Formation
US/CDS Findings
Increasingly inhomogeneous defect, hypoechoic structural alteration with eventually complex cystic configuration and rim-like margin; may resemble complicated cyst.
Focal perfusion defect, potentially capsular hyperaemia (Fig. 10.21); CEUS may enhance depiction and aid DDx.
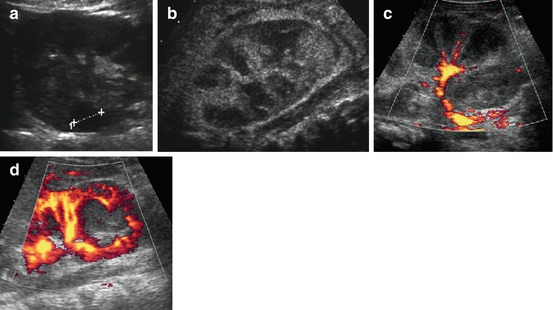

Fig. 10.21
Complications in UTI, DDx, scarring. (a) Focal necrotic cortical area (+….+) seen as hypoechoic sphaeric lesion in severely prolonged upper UTI; note also broadened peripyelonal echogenicity and hazy cortico-medullary differentiation of swollen kidney. (b) Multiple necrotic (dark) segmental defects throughout kidney in severe diffuse/multifocal necrotising pyelonephritis. (c) Same patient as in (b): aCDS demonstrates only minimal residual central perfusion – eventually entire kidney had to be removed. (d) Sphaeric focal complex liquid lesion in a child after UTI, with sparing of vessels on aCDS – consistent with early apperaence in formation of an renal abscess
10.3.2.4 Scarring
US/aCDS Findings
Regional narrowing of parenchyma with contour alteration, disrupted/abnormal cortico-medullary differentiation and clubbing of affected calix.
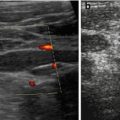
Regional small strip-like defect, particularly well seen on aCDS (Fig. 10.22).
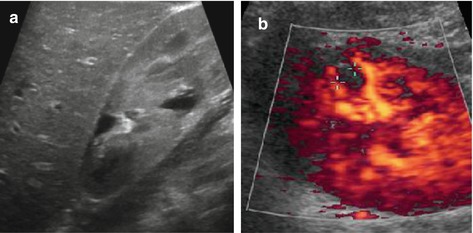
Fig. 10.22
Scarring. (a) Focal scar with clubbed calix and destroyed cortex after upper pole aPN. (b) Small peripheral scar (+ +) seen as perfusion defect on aCDS, not easily depictable by gray scale US
10.3.2.5 Tuberculosis
US/CDS Findings
Necrosis/atypical abscess – echogenic content, potentially with partial rim-like calcifications, commonly similar as complicated cyst. No other specific US/aCDS findings.
10.3.2.6 Xanthogranulomatous Pyelonephritis
Definition
Chronic infection, often with obstructive concretion/stone (“staghorn” shaped) that leads to destruction of medulla, eventually of entire kidney.
US/CDS Findings
Regionally or diffusely thinned parenchyma and vasculature (aCDS), peripheral hyperaemia in membrane around abscess formation. Pseudotumourous aspect of destroyed affected part. Disruption of normal renal parenchymal and vascular architecture in abscess and necrosis; potentially perifocal hyperaemia around kidney (Fig. 10.23).
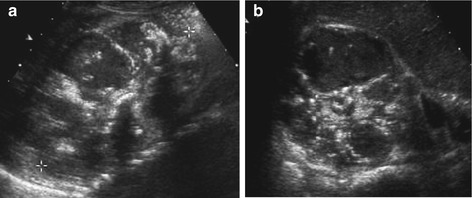

Fig. 10.23
Xanthogranulomatous pyelonephritis. Xanthogranulomatous pyelonephritis in a child with distal renal acidosis and stone disease, longitudinal (a) and axial (b) section: complex-cystic destruction of medullae, central calcifications, tumourously enlarged kidney, shape somewhat preserved
10.3.2.7 Glomerulonephritis/Nephrotic Syndrome
Definition
Large range of conditions.
US Findings
Commonly increased renal size, small medulla, enlarged cortex and potentially increased echogenicity/reduced cortico-medullary differentiation, depending on which compartments involved (Fig. 10.24).
Typically bilateral findings:
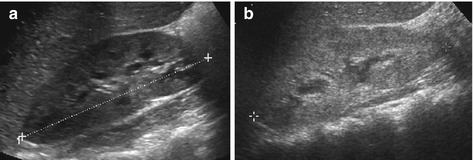

Fig. 10.24
Glomerulonephritis. (a) Swollen large right kidney (+ +) with small hypoechoic medullae in acute but mild (postinfectious) GN (findings bilateral, renal function normal). (b) A less-enlarged but still slightly swollen kidney with hyperechoic parenchyma and reduced cortico-medullary differentiation in chronic recurrent GN (HUS may appear similar)
In chronic stage, kidneys may be small.
In mild/atypical disease, kidney may look normal.
Differentiation between various entities sonographically impossible.
Other systemic findings: ascites, pleural effusion – depend on degree of renal failure/associated condition.
Consider renal manifestation of systemic disease such as lupus, Henoch-Schonlein purpura, amyloidosis, familiar Mediterranean fever, etc.
CDS Findings
Diffuse perfusion alterations – correlate more with degree of renal failure than with underlying entity (except for primarily vascular conditions – see below).
Role of US
Initial diagnosis: exclude other conditions by validating pre-/postrenal causes of renal failure. Assessment of renal perfusion, also during course of disease, as well as secondary/associated changes (e.g. under haemofiltration – intravascular volume?).
US-guided biopsy for histological evaluation (see Fig. 2.13).
10.3.3 Vascular Conditions
Role of US in Renal Vascular Conditions
Most developed tool for screening, initial diagnosis and and follow-up. US most useful for follow-up, differentiation against other renal conditions and monitoring of future renal growth.
Will allow depiction of changes in perfusion patterns:
However, if US potentially limited, then additional imaging necessary (see above).
Algorithms recommended for certain conditions, e.g. imaging in suspected childhood renovascular hypertension (see Pediatr Radiol (2010) 40:1315).
10.3.3.1 Renal Artery Stenosis
Definition
Rare in childhood, commonly not arteriosclerotic in origin but associated with other vasculopathies, after vasculitis, trauma/surgery, by compression.
US Findings
Using meticulous scanning techniques, entire course of extrarenal artery is often visualised.
Rarely changes in diameter or aneurysmal dilatation seen on gray scale.
Intrarenal portions only assessable using CDS.
CDS
Most striking finding: aliasing of colour spectrum – provided adequate scale settings.
Always perform spectral analysis:
At stenosis: marked increase in systolic velocity with spectral broadening + turbulent flow.
Distal to stenosis: decreased systolic velocity, more or less normal to elevated diastolic velocity, depending on severity/grade. Delayed systolic upstroke with increased acceleration index (pulsus tardus et parvus) (Fig. 10.25).
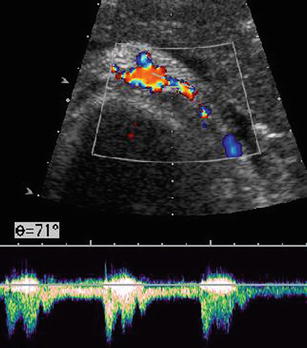
Fig. 10.25
Renal artery stenosis. Elevated flow velocity with turbulent atypical flow at site of left renal artery stenosis, also seen as aliasing of CDS signals and narrow vessel diameter
In kidney: sometimes aCDS depicts segmental hypoperfusion due to infarction of affected area after severe stenosis.
NOTE: Always assess all renal sectors (as in children intrarenal stenosis quite common) – peripheral and main segmental branches need to be seen on CDS for reliable assessment. Always assess abdominal aorta/other low-resistive flow vessels (e.g. coeliac trunk, cerebral vessels) to differentiate focal from systemic conditions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree