CHAPTER 4 Unusual and Problematic Types of Breast Cancers: DCIS, Intracystic Papillary Carcinoma, Benign-appearing Breast Cancers, ILC, Inflammatory Breast Cancer, and Breast Cancer in Implant Patients
Certain subtypes of breast cancer can be particularly challenging to detect on routine mammography. This can have implications for staging and surgical outcomes.1 Although the use of supplemental imaging tools, such as ultrasound and MRI, help to improve cancer detection and delineation of the extent of disease,2 cancers such as invasive lobular carcinoma (ILC) and ductal carcinoma in situ (DCIS) continue to be problematic. Other generally readily detected carcinomas, such as medullary, papillary, and mucinous (colloid), may be difficult to recognize as malignant because of their propensity for relatively benign-appearing morphologic features.
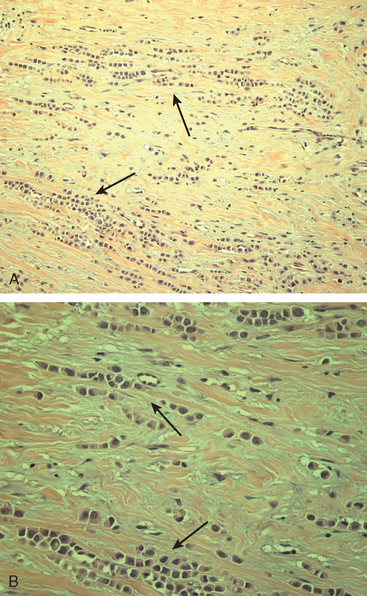
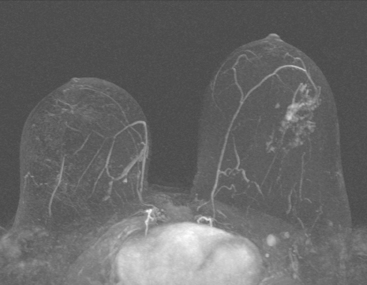
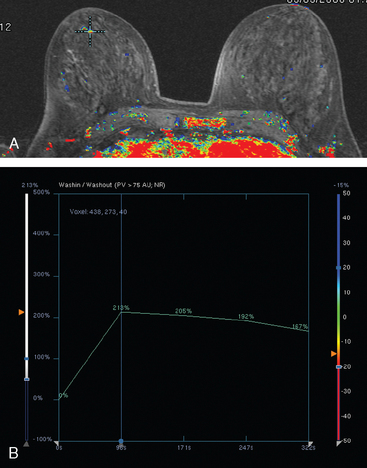
Although ILC represents only about 10% of all breast tumors,3 it is known to be one of the most common reasons for a false-negative mammogram.4 The infiltrating growth pattern of single-file strands of malignant cells, often with minimal fibrotic reaction, is one of the reasons that ILC can be difficult to detect (Figure 1). In addition, if an ILC does produce a mammographically detectable finding, it may not form a mass and may be of relatively low or equal density to normal fibroglandular tissue.3 Even large lesions may still be occult on mammography.5 Mammographic sensitivity for ILC ranges between 57% and 89%.4–8 In addition, ILC has a higher propensity for multifocal and bilateral involvement. Its extent is often underestimated by mammography.9,10 Understaging can significantly affect surgical outcomes and patient treatment. MRI has been shown to be useful in better defining the extent of disease in patients with ILC.10,11
The increasing use of screening mammography has led to an increase in the detection of DCIS, usually presenting as clustered microcalcifications. However, establishing the extent of disease can be problematic because DCIS is commonly multifocal and is often noncalcified. A recent study suggested that MRI may be more useful in detecting DCIS than previously thought.12
Mucinous carcinoma, also termed colloid carcinoma, is relatively uncommon.13 Because it often presents as a circumscribed mass, it may potentially be misinterpreted as a benign lesion, such as a fibroadenoma. However, close inspection usually reveals features that should distinguish mucinous carcinoma from benign entities, such as marginal irregularity or heterogeneous echotexture on ultrasound. In a similar manner, medullary carcinoma can present as a well-circumscribed mass. Medullary carcinomas account for about 3% to 5% of breast cancers and have a prognosis that is generally better than more common types of invasive breast cancer.
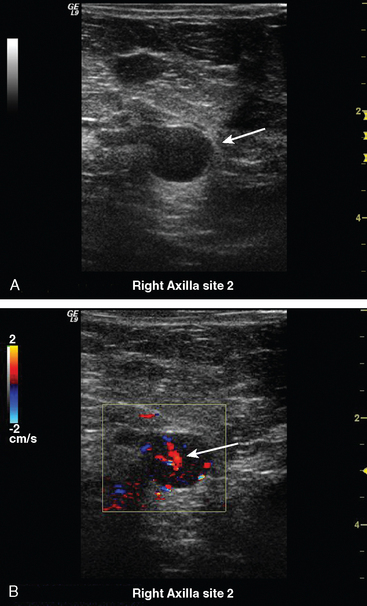
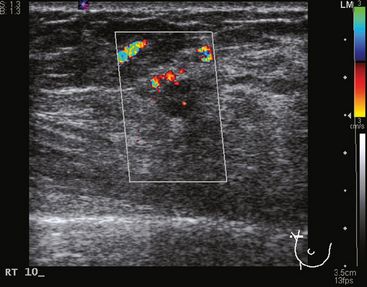
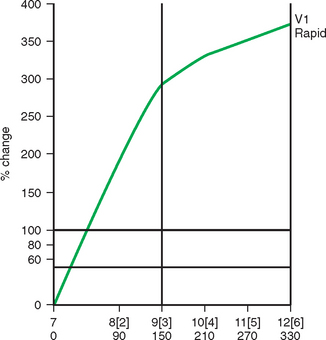
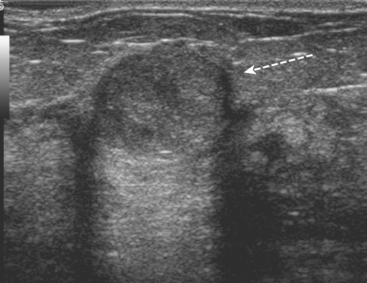
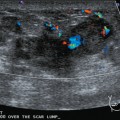
Some carcinomas may have features on ultrasound that could be confused with benign entities. Purely hyperechoic lesions on ultrasound, such as a lipoma, are invariably benign. However, some invasive carcinomas may have a hyperechoic halo that may simulate a benign lesion. On close inspection, a hypoechoic “nidus” or central region is generally present to distinguish carcinomas from completely hyperechoic benign lesions. Some carcinomas, particularly high-grade cancers and metastatic lymph nodes, may be extremely hypoechoic on ultrasound and could be mistaken for anechoic cysts. In addition to proper gain settings and margin analysis, color Doppler helps in distinguishing solid masses from cysts (Figure 2).
1 Veltman J, Boetes C, van Die L, et al. Mammographic detection and staging of invasive lobular carcinoma. Clin Imaging. 2006;30(2):94-98.
2 Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830-849.
3 Newstead GM, Baute PB, Toth HK. Invasive lobular and ductal carcinoma: mammographic findings and stage at diagnosis. Radiology. 1992;184(3):623-627.
4 Krecke KN, Gisvold JJ. Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am J Roentgenol. 1993;161(5):957-960.
5 Holland R, Hendriks JH, Mravunac M. Mammographically occult breast cancer: a pathologic and radiologic study. Cancer. 1983;52(10):1810-1819.
6 Hilleren DJ, Andersson IT, Lindholm K, Linnell FS. Invasive lobular carcinoma: mammographic findings in a 10-year experience. Radiology. 1991;178(1):149-154.
7 Paramagul CP, Helvie MA, Adler DD. Invasive lobular carcinoma: sonographic appearance and role of sonography in improving diagnostic sensitivity. Radiology. 1995;195(1):231-234.
8 Le Gal M, Ollivier L, Asselain B, et al. Mammographic features of 455 invasive lobular carcinomas. Radiology. 1992;185(3):705-708.
9 Lee JSY, Grant CS, Donohue JH, et al. Arguments against routine contralateral mastectomy or undirected biopsy for invasive lobular breast cancer. Surgery. 1995;118:640-648.
10 Boetes C, Veltman J, van Die L, et al. The role of MRI in invasive lobular carcinoma. Breast Cancer Res Treat. 2004;86(1):31-37.
11 Mann RM, Veltman J, Barentsz JO, et al. The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. Eur J Surg Oncol. 2008;34(2):135-142. Epub 2007 Jun 15.
12 Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370(9586):485-492.
13 Dhillon R, Depree P, Metcalf C, Wylie E. Screen-detected mucinous breast carcinoma: potential for delayed diagnosis. Clin Radiol. 2006;61(5):423-430.
CASE 1 DCIS, calcified and noncalcified
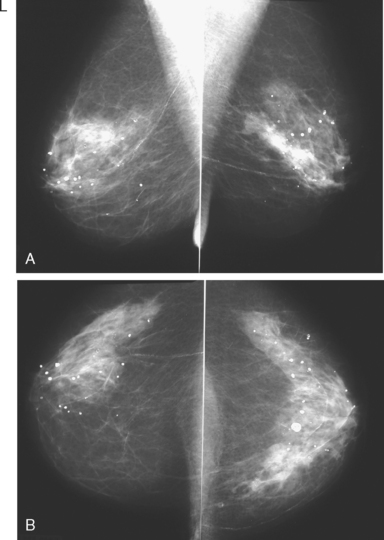
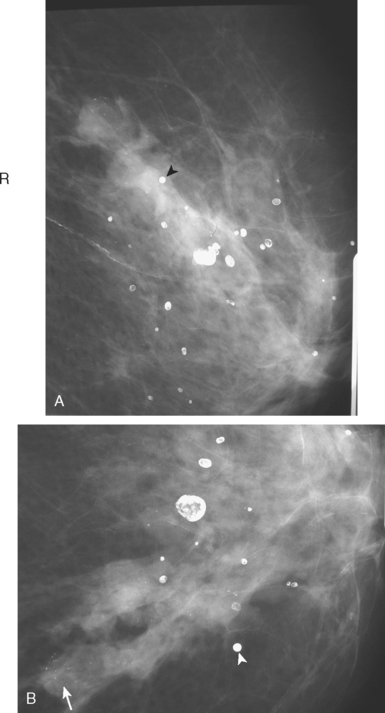
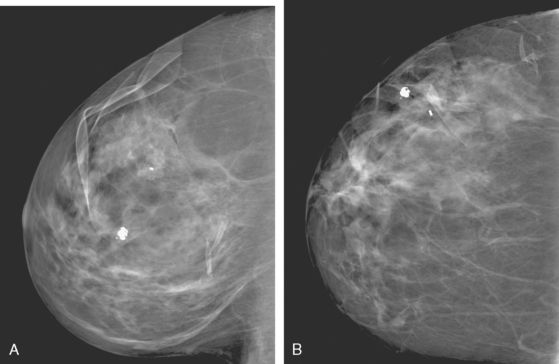
A 50-year-old woman was found on screening mammography to have suspicious pleomorphic microcalcifications in the 12-o’clock position of the right breast (Figure 1). Biopsy was performed with stereotactic technique, confirming intermediate-grade ductal carcinoma in situ (DCIS) (Figure 2). There was a family history of breast cancer, most notably in a sister at age 31.
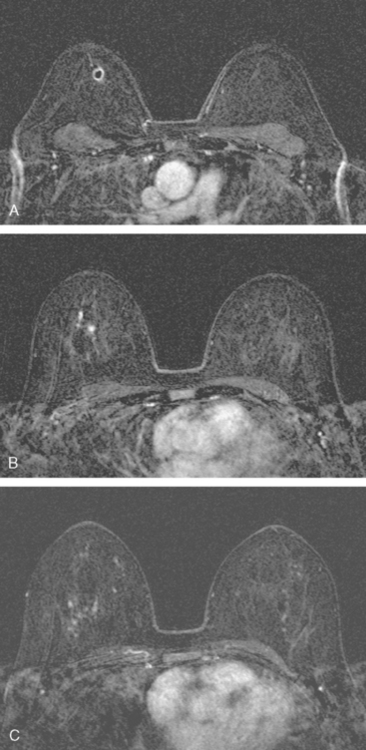
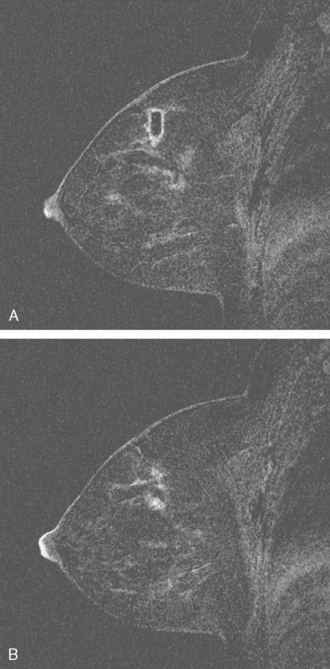
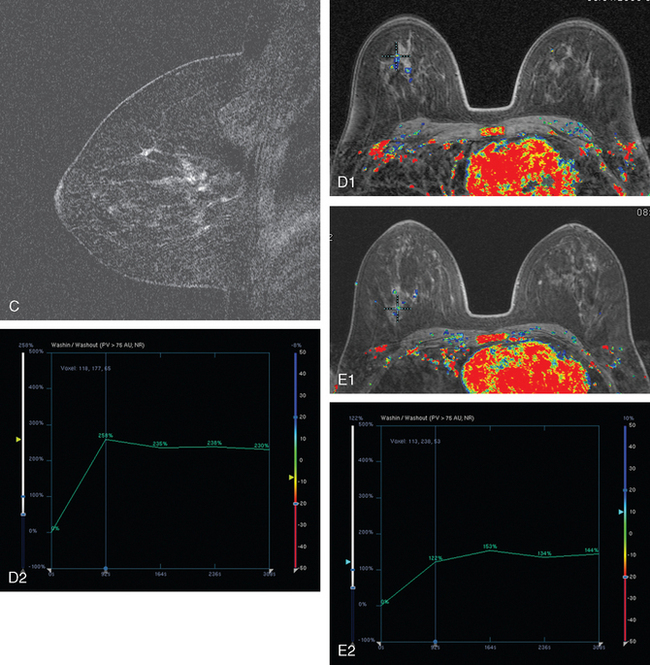
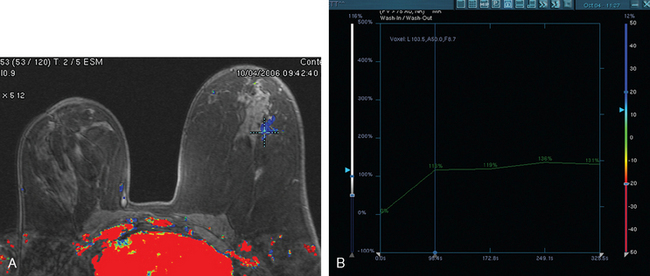
Breast MRI was obtained to evaluate for occult invasive components and extent of disease. Ultrasound had not shown an associated mass. The breast MRI showed clumped, small masses of intense enhancement with washout at the expected level of the residual known DCIS (Figures 3 and 4). There was a second, separate site of concerning, clumped enhancement in the same breast, with plateauing enhancement, thought suspicious for possible additional, noncalcified DCIS. MRI-guided biopsy was performed, and pathology showed two tiny foci of high-grade DCIS, the largest 1 mm in size. A clip was placed to mark the MRI-guided biopsy site, and postbiopsy mammography confirmed it to be removed in location from the remaining microcalcifications (Figure 5).
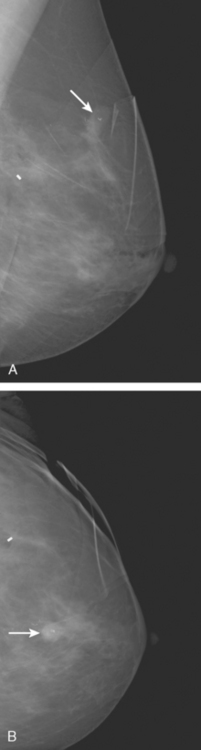
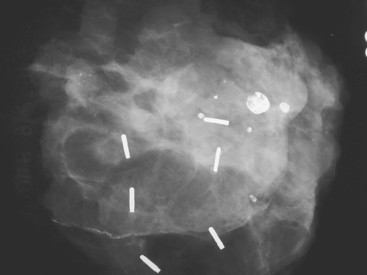
These evaluations showed the patient had proven multicentric DCIS, including noncalcified DCIS. She was recommended to have a mastectomy but was strongly desirous of breast conservation. Accordingly, lumpectomies were performed after a triple-needle localization, in which the remaining microcalcifications at 12 o’clock were bracketed with two needles (Figure 6), and the clip at 9 to 10 o’clock from the MRI-guided biopsy was separately localized (Figure 7). The specimen at 12 o’clock contained both of the bracketing localization wires and the remaining pleomorphic microcalcifications. These were noted to approach a margin, from which additional tissue was obtained. The initial specimen from the 9- to 10-o’clock localization showed the hook wire, but not the MRI-placed clip. The surgeon was advised of this, additional tissue was obtained and radiographed, and the clip was found in the second specimen (Figure 8).
Mastectomy was again recommended for this patient, who desired re-excision and breast conservation. The 12-o’clock lumpectomy site was re-excised. The re-excised lateral margin showed three microscopic foci of high-grade DCIS (largest, 0.5 cm), two of which were closer than 2 mm to the new lateral margin. The new inferior margin was clear, and this specimen showed a single microscopic focus of DCIS. The additional deep margin excision showed three foci of invasive ductal carcinoma (IDC) (largest, 0.4 cm), with multifocal high-grade DCIS involving greater than 50% of the lesion (extensive intraductal component), DCIS focally at the new deep margin, and multiple foci of DCIS and IDC closer than 2 mm to the new deep margin.
CASE 2 Extensive intraductal carcinoma presenting as a palpable, tumor-filled ductal system
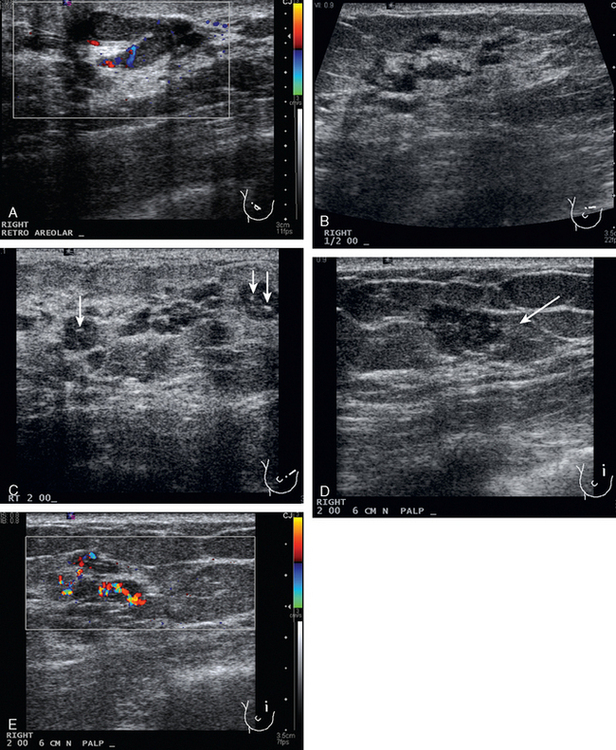
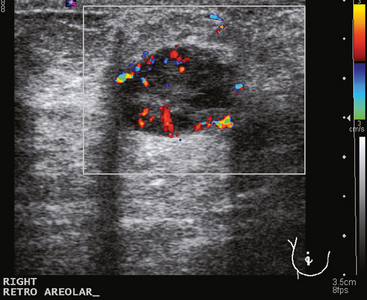
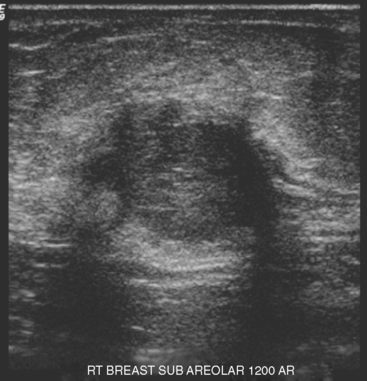
A 74-year-old woman was evaluated for right upper inner quadrant (UIQ) palpable firmness. Mammography showed a segmental region of tubular nodularity with suspicious microcalcifications, spanning 5 cm, extending from the retroareolar region to the UIQ (Figures 1 and 2). On ultrasound, dilated retroareolar soft tissue containing ducts extended into the UIQ (Figure 3). Ultrasound-guided biopsy obtained intermediate-grade intraductal carcinoma with papillary and cribriform features. The patient was a part-time resident of the area and had multiple medical problems, including severe atherosclerotic disease with prior coronary artery bypass graft, stents, and abdominal aortic aneurysm repair. She elected treatment with partial mastectomy and interstitial brachytherapy (Figure 4).
CASE 3 BRCA1 patient, abnormal whole-body PET leading to diagnosis of DCIS
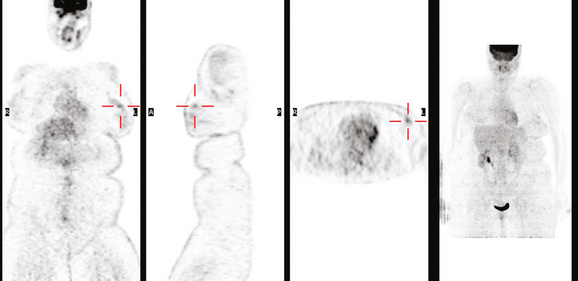
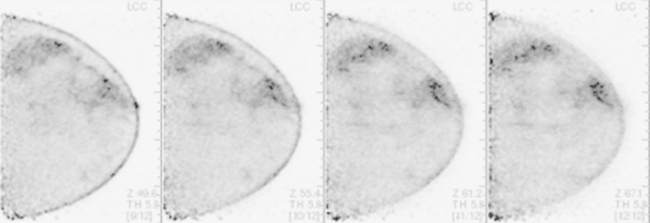
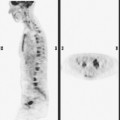
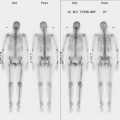
A 53-year-old woman with a past medical history of right breast cancer 15 years before and ovarian cancer 5 years previous, underwent positron emission tomography (PET)/CT for surveillance of ovarian cancer. Her prior breast cancer was infiltrating ductal carcinoma (IDC), which had been treated with lumpectomy and radiation. Whole-body PET/CT showed asymmetrical, relatively focal, mildly increased uptake in the left lateral breast (Figure 1). Correlation with a recent mammogram showed no corresponding abnormality. Left breast ultrasound was also negative.
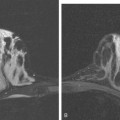
Breast MRI was obtained 6 months later. Segmental, clumped, plateauing enhancement was found in the left lateral breast (Figures 2, 3, and 4). A positron emission mammography (PEM) scan with fluorodeoxyglucose (FDG) was obtained as well (Figures 5 and 6).
MRI-guided biopsy was performed (Figure 7). Pathology showed high-grade ductal carcinoma in situ (DCIS), with comedonecrosis and cribriform types, and lobular cancerization and multiple foci suspicious for microinvasion. The tumor was estrogen receptor and progesterone receptor negative.
TEACHING POINTS
The patient’s high-grade DCIS was picked up as an unsuspected finding on whole-body PET scan, obtained for surveillance for ovarian cancer. Initial workup with mammography and sonography showed no correlate. Breast MRI is the appropriate next breast imaging step in the evaluation, given the patient’s high risk profile and the unexplained PET scan finding.
As of this writing, PEM devices have only recently become available and are limited in distribution. There is little collective experience with the capabilities and limitations of PEM scanning. A multicenter prospective trial comparing the performance of PEM to breast MRI in preoperative staging of newly diagnosed breast cancers is accruing patients, and data from this trial will hopefully help in delineating the appropriate role of PEM in the breast imaging armamentarium.
CASE 4 Intracystic papillary carcinoma
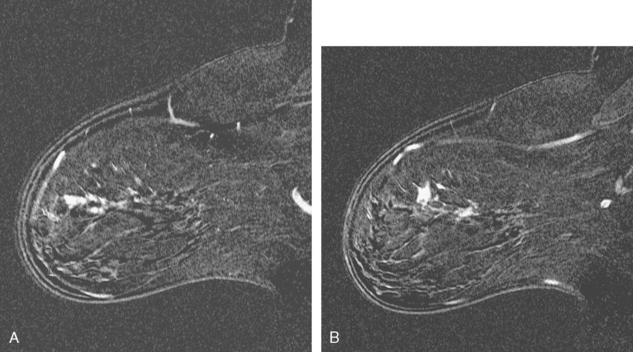
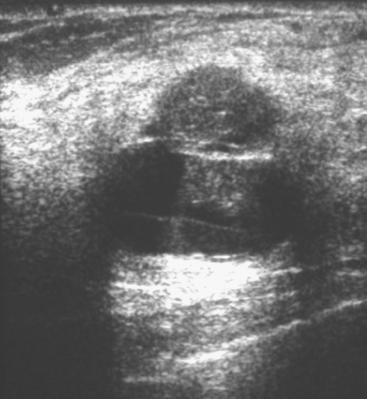
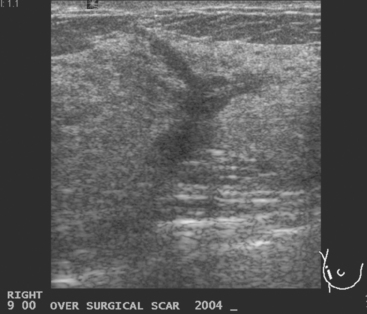
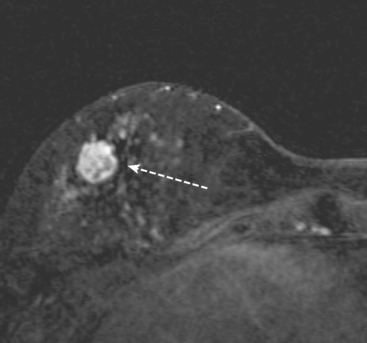
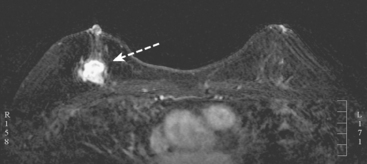
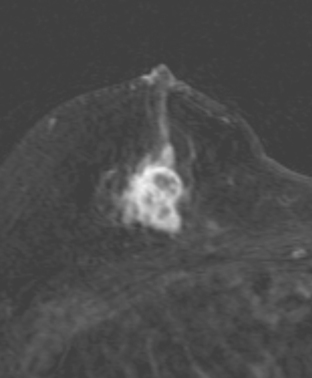
A 51-year-old woman underwent breast imaging evaluation for a clear right nipple discharge. Two years before, she was diagnosed with right breast intracystic papillary neoplasm, estrogen receptor positive (Figure 1), and treated with lumpectomy only. On physical examination, a serous fluid discharge could be readily elicited by palpation of the right lateral breast, in the region of her surgical scar. No clear imaging correlate could be identified on diagnostic mammography or breast ultrasound, with scarring noted on ultrasound at the lumpectomy site (Figure 2). Breast MRI was obtained and showed an unusual focus of branched, linear right retroareolar enhancement, with washout (Figures 3 and 4). A second focus of abnormal mass enhancement measuring 8 mm was identified, at the 9-o’clock right breast posterolateral level, with washout (Figure 5). MRI-guided biopsy was performed of the retroareolar branched enhancement, identifying intracystic papillary carcinoma, considered in situ, with no invasion identified. The second site was too posterior to reach with a grid MRI-localizing device. A second breast ultrasound was performed to find a correlate for the posterior MRI abnormality (Figure 6). A 6-mm hypoechoic nodule was identified on ultrasound at 10 o’clock, thought to be the probable correlate for the MRI finding. This had not been noted on a prior right breast ultrasound, obtained before the MRI. Ultrasound-guided core needle biopsy identified low-grade, cribriform ductal carcinoma in situ (DCIS), similar in histology to the patient’s prior specimens.
With two proven sites of right breast intracystic papillary carcinoma (IPC)/DCIS, the patient was treated with modified radical mastectomy. She elected to undergo prophylactic mastectomy on the left at the same time, with immediate reconstruction with tissue expanders. On the right, one sentinel and one additional lymph node were negative. The right mastectomy specimen showed focal residual intracystic papillary carcinoma and focal low-grade micropapillary DCIS at the subareolar level. The left prophylactic mastectomy specimen was negative.
An additional example of this less common histology is illustrated in Figure 7.
TEACHING POINTS
This patient’s original IPC shows typical imaging features of these rare lesions, with a complex cyst with septations and solid components. These lesions can vary in appearance from predominantly cystic (often complex, with septations and mural nodules, as here) to predominantly solid (another example, different patient, Figure 7).
Associated DCIS or invasive cancer is found 40% of the time, and there is potential for axillary nodal involvement. These tumors are often estrogen receptor positive. The prognosis for IPC without DCIS or infiltrating ductal carcinoma is excellent. No influence on recurrence or survival using radiation therapy has been demonstrated.
CASE 5 Colloid cancer, two cases
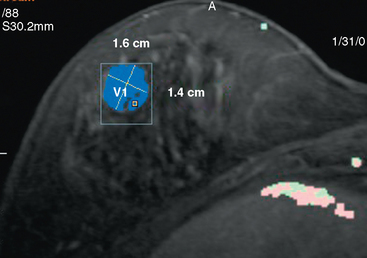
A 46-year-old woman, with a known left breast sarcoma history, underwent preoperative bilateral MRI. The MRI demonstrated a previously unknown mass in the contralateral breast (Figures 1, 2, 3, and 4). Ultrasound identified a solid corresponding mass in the lower outer right breast (Figure 5). Ultrasound-guided core needle biopsy diagnosed an invasive mucinous carcinoma. The patient was treated with bilateral lumpectomies and radiation therapy.
TEACHING POINTS
This case is an example of a mucinous carcinoma, also termed colloid carcinoma, which consists of tumor cells floating within pools of mucin. On imaging, mucinous carcinomas potentially can be misinterpreted as benign. Features suggestive of benignity illustrated by this lesion include relatively well circumscribed margins, high T2 signal on MRI, and posterior acoustic through-transmission on ultrasound. However, detailed examination helps to prevent confusing this lesion for a fibroadenoma. Close inspection of both the MRI and the ultrasound shows that the margins of the mass are not as well circumscribed as expected for a fibroadenoma. In addition, unlike classic fibroadenomas, the internal architecture of this mucinous carcinoma is heterogeneous on both the MRI and ultrasound. Mammographically, mucinous carcinomas often present as wellcircumscribed, relatively low-density masses, which may delay their diagnosis. Pure mucinous carcinoma is an uncommon form of breast malignancy, accounting for 1% to 2% of all breast cancers. They tend to occur in older patients. In contrast to mixed mucinous carcinomas, pure mucinous carcinomas have a favorable prognosis, are often low-grade tumors, and rarely metastasize.
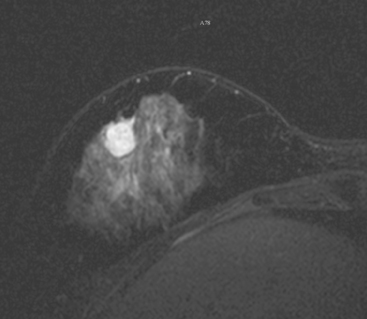
Not every colloid carcinoma is as innocent in appearance as this example. Another case of an invasive mucinous carcinoma, in a 72-year-old woman with a palpable right breast mass, also shows bright signal on T2-weighted MRI, but other features of malignancy are notable (Figures 6, 7, 8, and 9).
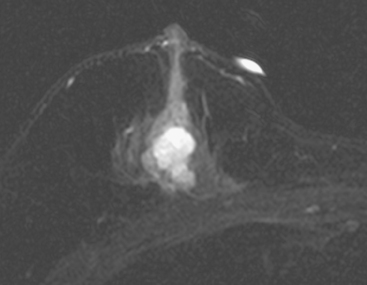
FIGURE 8 MR T2-weighted spin-echo image of the right breast. The mass demonstrates high T2 signal intensity.
TEACHING POINTS
This case demonstrates a less benign imaging appearance of a mucinous carcinoma than the preceding case. The bright signal on T2-weighted sequences might lead one to consider a fibroadenoma in the imaging differential diagnosis. However, the heterogeneity of the enhancement, with rim and internal septation enhancement, and the marginal irregularity, are features highly suggestive of malignancy. As always in breast imaging, a lesion should be judged by the most sinister feature it displays, and one should not be reassured by other, more typically benign characteristics.