Rakesh C. Navuluri • Brian Funaki
Approximately 100,000 cases of upper gastrointestinal bleeding require inpatient admission annually in the United States. When medical management and endoscopic therapy are inadequate, endovascular intervention can be lifesaving. This chapter begins with a brief review of the preangiographic workup of patients with upper gastrointestinal bleeding. This will be followed by a detailed discussion of the angiographic technique, including a closer look at the use of various embolic agents. The primary focus will be on nonvariceal (arterial) hemorrhage with brief consideration given to variceal (venous) hemorrhage.
BACKGROUND
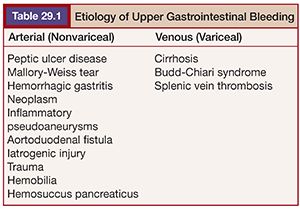
Upper gastrointestinal bleeding (UGIB) is defined by a bleeding source proximal to the ligament of Treitz. UGIB accounts for 76% of gastrointestinal (GI) bleeding events.1 The incidence of UGIB in the United States is estimated at 102 per 100,000 per year, with a mortality rate of 5% based on data from 1991.2 UGIB can be divided into arterial (nonvariceal) and venous (variceal) etiologies. This is an important point of differentiation and a major branch in the management decision tree. Causes of arterial UGIB include peptic ulcer disease (up to 40%), Mallory-Weiss tear (15%), hemorrhagic gastritis, pancreatitis-related pseudoaneurysms, neoplasm, aortoduodenal fistula, and trauma. Other rare causes of arterial UGIB include hemobilia from iatrogenic injury and hemosuccus pancreaticus related to chronic pancreatitis. Venous causes include variceal bleeding secondary to portal venous hypertension (e.g., due to cirrhosis or Budd-Chiari syndrome) or splenic vein thrombosis (Table 29.1).
Symptoms
Typical UGIB symptoms include hematemesis or melena. Hematochezia, although commonly attributed to lower GI sources, can also occur depending on the bleeding rate and transit time of blood through the bowel. Patients with nonvariceal bleeding commonly present with coffee-ground emesis and history of nonsteroidal anti-inflammatory drug use. Variceal bleeding is likely to present with clinical signs of cirrhosis, painless hematemesis, and a greater degree of hemodynamic instability.
Algorithm
Current treatment algorithms call for immediate medical stabilization followed by endoscopic diagnosis and intervention. Refractory cases should be referred for either transvenous or transarterial endovascular intervention, depending on the source of bleeding identified at endoscopy. Although surgery is generally considered the last option, endovascular intervention may be reattempted following failed surgery.
Medical
Stabilization of blood pressure is the immediate objective in managing patients with UGIB. Fluid resuscitation should be instituted without delay. Patients with hemodynamically significant bleeding should also be immediately typed and crossed to allow for transfusion of blood products as necessary. The goal of transfusion in this setting is to raise the hemoglobin level above 7 g/dL. Any existing coagulopathy (international normalized ratio [INR] >1.5 or platelet count <50,000/µL) should also be corrected.3 It is important to note that units of packed red blood cells (pRBCs) do not contain the factors and platelets needed for thrombogenesis. If these are not replaced during large-volume blood transfusions, a dilutional coagulopathy will result, which may affect the success of subsequent embolization procedures.
Insertion of a nasogastric tube can help to confirm an upper GI source of bleeding. If variceal bleeding is suspected, a Minnesota tube (or Sengstaken-Blakemore tube) can be placed to tamponade esophageal varices. It has been reported to have variable success in achieving hemostasis but is a good option in stabilizing patients in the emergent setting before interventional procedures.
An estimated 10% to 15% of cases require intervention beyond medical management to control bleeding.4 To help sustain the success of subsequent interventional procedures, various medication regimens may be instituted. Administration of proton pump inhibitors (PPIs) have been shown to reduce the rate of rebleeding and surgery in high-risk patients with nonvariceal UGIB.5 This is because hemostasis within the stomach and duodenum is impeded by low intragastric pH. Low pH has been shown to inhibit platelet function and activate pepsin, which disaggregates platelet plugs.6 The use of portal pressure–reducing medications such as somatostatin, octreotide, or vasopressin can improve outcomes in patients with variceal UGIB.7
Endoscopy
Endoscopy should be the initial intervention as it allows for localization of bleeding and determination of etiology via visual inspection and biopsy. It can be particularly helpful in differentiating arterial and venous sources of hemorrhage. Endoscopy can also identify upper and midesophageal sources of bleeding that are not amenable to embolization such as esophagitis. Beyond its diagnostic use, endoscopy also offers several therapeutic options. Hemostasis can be achieved endoscopically via thermocoagulation, sclerosant injection, or clips (banding). Endoscopic clip placement, even if ineffectual, can aid subsequent endovascular intervention by directing the interventional radiologist to the area of concern.
Endoscopy performed within 24 hours of presentation is associated with improved patient outcome and decreased hospital stay. However, the source of bleeding may not be seen in up to 24% of patients at the first endoscopic exam.8 Although endoscopic treatment is reported to be effective in 85% to 90% of patients,9 repeat bleeding occurs in 15% to 20%, most commonly in the first 72 hours after treatment.10 Endoscopic predictors of rebleeding include visualized active bleeding, nonbleeding visible vessel, adherent clot, ulcer size greater than 2 cm, and ulcer location in the posterior midgastric body or posterior duodenal bulb11–13 (Table 29.2). Recurrent bleeding should be followed with a second endoscopic treatment before endovascular intervention is undertaken.14 Further bleeding after a second endoscopic treatment should be referred to interventional radiology. Alternatively, the inability to visualize the bleeding source at endoscopy due to the severity of bleeding warrants urgent endovascular intervention.

Localization
Localization of bleeding before angiography is important for several reasons. First, it decreases procedure time by allowing for a more targeted approach. This consequently reduces radiation exposure as well as contrast load used during angiography. Minimizing contrast dose is critical in patients with borderline azotemia. Second, it has also been shown to directly impact the success rate of endovascular therapy.15 Last, it permits empiric embolization to be undertaken even if active bleeding is not seen at angiography.
If the source of bleeding cannot be identified, angiography may be deferred. However, in the setting of hemodynamic instability or in patients requiring more than 5 units of pRBCs, angiography should be performed immediately. In these circumstances, angiography is the most practical diagnostic tool because it offers the option of concomitant therapy.16 If the clinical situation permits, localization with computed tomography angiography may be considered before proceeding to conventional angiography.
Computed Tomography Angiography
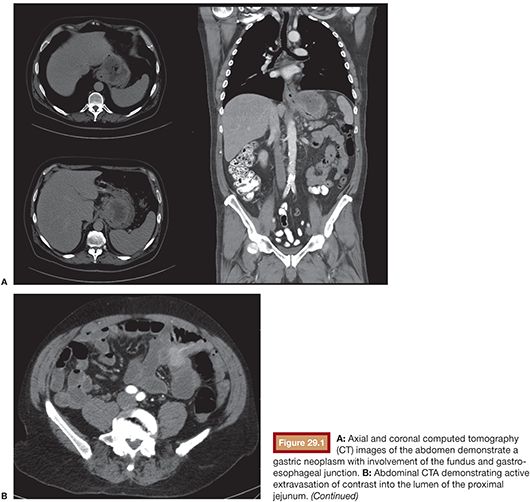
Compared with conventional angiography, computed tomography angiography (CTA) has the advantage of greater availability, speed, and noninvasiveness. It also does not suffer from bowel or respiratory motion artifact. In an animal model, CTA has been shown to detect bleeding rates as low as 0.3 mL per minute—better than conventional angiography.17 No studies specific to UGIB have been performed to date, but a study by Yoon et al.18 found CTA to have a sensitivity of 90.9%, a specificity of 99%, and an accuracy of 97.6% in localizing hemorrhage when considering both upper and lower GI sources. CTA, unlike conventional angiography, may also detect lesions, such as ulcers, masses and aneurysms, that are not bleeding at the time of the study. The ability to characterize the inciting lesion can have a significant impact on treatment planning. If, for example, a mass lesion is seen, particle embolization may be performed where it might otherwise be avoided in the treatment of GI hemorrhage to avoid bowel necrosis. CTA also has the benefit of providing information on the vascular anatomy for preinterventional planning. For example, a steep takeoff of the celiac artery may necessitate the use of a Waltman loop or even brachial artery access. Stenotic or tortuous mesenteric arteries indicate that a guiding catheter will be helpful. Similarly, occlusion of the celiac axis may require embolization of the pancreaticoduodenal arcade via the superior mesenteric artery (SMA) (Fig. 29.1).
CTA protocols are similar to those for endoleak evaluation. A noncontrast series is followed by intravenous contrast-enhanced series in arterial and delayed phases. A positive CTA study will show hyperdense contrast material (>90 HU) within the bowel lumen on the arterial phase that increases on the delayed phase.19 This provides up to a 90-second window of time to evaluate for contrast extravasation—depending on the timing of the delayed series—compared with a 10-second window of evaluation for conventional angiography. Oral contrast should not be given during the exam because it will obscure intravenous contrast extravasation into the bowel lumen.
The primary drawback of CTA is the necessity of intravenous iodinated contrast, which may elicit a hypersensitivity reaction or cause renal injury.20 Typically, 1.5 mL/kg (up to 150 mL) is administered. This can be prohibitive in patients with poor renal function, particularly when considering the additional contrast required in a subsequent endovascular procedure. Radiation dose is another drawback to consider.
Nuclear Medicine
Radionuclide scintigraphy may be helpful in identifying sources of chronic GI bleeding, which are otherwise not detectable by endoscopy or conventional angiography. Technetium 99m (Tc-99m)–labeled red blood cell scans can detect bleeding rates as low as 0.2 mL per minute, compared with 0.5 mL per minute for angiography, and can be particularly useful in the setting of intermittent GI bleeding. Unlike angiography and CTA, bleeding scans allows for interrogation over a multiple-hour window, which increases sensitivity for detection of GI bleeding. In patients with hemodynamic instability, studies with an immediate tracer blush are more likely to have a positive angiogram than studies with a delayed blush.21 It is worth noting that positive scintigrams can also occur in cases of variceal bleeding (Fig. 29.2).
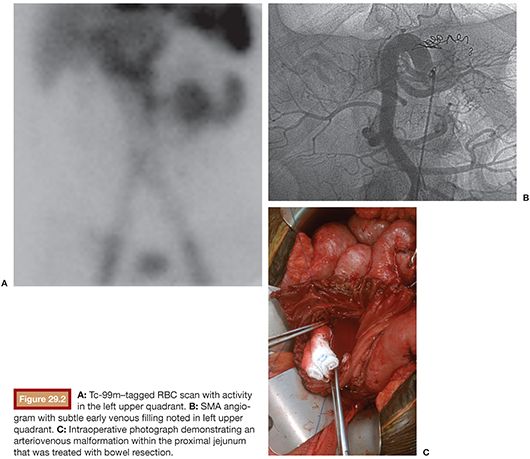
In terms of localizing bleeding within the upper GI tract, nuclear medicine is limited by the low resolution of imaging as well as confounding factors such as uptake of free technetium by the gastric mucosa. Consequently, at our institution, we do not routinely recommend radionuclide scintigraphy for localizing the site of bleeding in the upper GI tract.
Once active hemorrhage is documented and localized with a diagnostic radiology study, patients should be transferred immediately to the angiography suite. At our institution, we strive to perform angiography within 1 hour of radiologic diagnosis of active GI bleeding.
Angiography Indications
The primary indication for conventional angiography is the inability to control the source of bleeding endoscopically. Angiography is favored over surgery as the treatment of choice after failed endoscopic therapy, particularly in high-risk surgical patients.16 It is minimally invasive, associated with lower mortality,22 and there has been shown to be no difference in outcome between patients managed with surgery versus arterial embolization.23,24
Angiography is also warranted in instances where the bleeding site cannot be identified by endoscopy or CTA and the patient is hemodynamically unstable as it may be lifesaving. Endovascular embolization is also the primary treatment for hepatobiliary bleeding.
Preangiography
Before angiography, the patient’s renal and coagulation statuses should be assessed. Elevated partial thromboplastin time and prothrombin time/INR as well as thrombocytopenia should be corrected. Embolization is less likely to succeed in the setting of coagulopathy because the most common embolic agent used—coils—causes vessel obstruction by providing a scaffold for thrombus formation rather than by pure mechanical occlusion.16 Furthermore, several studies have demonstrated preinterventional coagulopathy to have a negative effect on clinical success.25,26 Embolotherapy has been reported to be nearly three times more likely to fail in patients with coagulopathy.27 If necessary, blood products may be given intraprocedurally to expedite the procedure.
Even in the setting of uncorrectable coagulopathy, embolization often remains the best option for treatment. In these scenarios, alternative embolic agents including placement of a Gelfoam sandwich or the application of glue or Onyx (Micro Therapeutics Inc., Irvine, California) may prove effective.
ANGIOGRAPHY AND EMBOLOTHERAPY
Review of imaging, such as CTA, before intervention can profoundly expedite cases by demonstrating vascular occlusions or variant anatomy. One should also consider the angle at which the mesenteric vessels arise from the aorta.
The right common femoral artery is the default access site for mesenteric angiography. We begin with a mapping aortogram to obtain a survey of the vascular anatomy. This is helpful in identifying the ostia of the mesenteric vessels and in guiding subsequent catheter selection. This step takes only a few minutes to accomplish and can save valuable time later on in the procedure. If recent imaging of the vascular anatomy is available, either as a CTA or conventional angiogram, an initial aortogram can be skipped in favor of a selective mesenteric angiogram to decrease contrast load in patients with poor renal function or to expedite the procedure. Contrast extravasation is rarely visible on the mapping aortogram. We use a 5-Fr nonmarking pigtail catheter (Cook Medical Inc., Bloomington, Indiana) placed through a 5-Fr vascular sheath. The pigtail should be positioned just below the level of the diaphragm. Power injection is performed at a rate of 20 mL per second, for a total volume of 30 mL.
Although not required, 1 mg glucagon can be administered before angiography to limit peristalsis motion artifacts on digital subtraction angiography (DSA). For similar reasons, breath hold during DSA is ideal to limit respiratory motion. However, this is not always feasible depending on the patient’s condition and level of sedation.
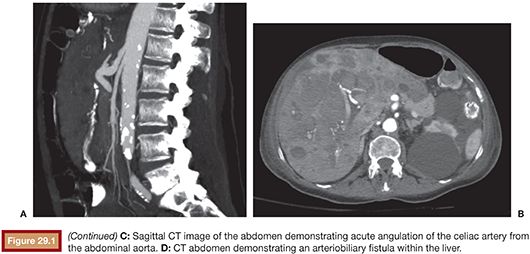
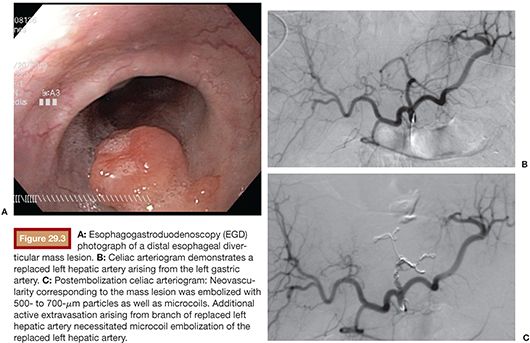
We favor the use of Rosch celiac (RC-1) or visceral selective (VS1) catheters (Cook Medical Inc., Bloomington, Indiana) to select the celiac artery and SMA. It is important to seat the catheter just beyond the ostium of the mesenteric vessel. If advanced too far, early branching vessels may not be imaged on the angiogram. Power injection of contrast is used for all angiograms to optimize detection of active bleeding. Celiac artery and SMA angiogram injection rates are typically 5 mL per second, for a total volume of 20 to 25 mL. DSA is carried out until the portal venous phase to document patency of the portal vein. This can be important to document in cases that potentially require hepatic artery embolization (Fig. 29.3). Delayed angiograms may also reveal varices that were not readily apparent by endoscopy. If there is hemobilia related to recent percutaneous biliary drain placement, removal of the tube over a guidewire may be necessary before angiography as the relative tamponade effect of the tube may obscure visualization of the bleeding vessel.
If no evidence of bleeding is found at celiac angiography, superselective catheterization of the suspected second-order branch (gastroduodenal artery [GDA] or left gastric) is undertaken. A microcatheter is preferred in these circumstances to avoid inducing vasospasm before the culprit vessel is identified. If these studies are also negative, an SMA arteriogram is performed before terminating the exam.
Angiography can detect bleeding rates as low as 0.5 mL per minute. The primary angiographic findings of bleeding are visualization of active contrast extravasation and contrast pooling in the venous phase. A review of studies by Loffroy et al.28 found that angiographic evidence of active extravasation was seen in 54% of cases. Other indirect signs of bleeding on angiography include pseudoaneurysm, vessel spasm or cutoff, early venous filling, and hypervascularity (Table 29.3).

The presence of an abnormal blush may indicate an inflammatory process. This can represent a bleeding source if such an entity was suspected on prior endoscopy.28 In cases of hemorrhagic neoplasm, tumoral blush and neovascularity may be identified. Not uncommonly, trial subselection of vessels is necessary to demonstrate bleeding.
In theory, carbon dioxide (CO2) angiography is more sensitive than conventional angiography with iodinated contrast because the lower viscosity of CO2 should predispose it to extravasating through endothelial injuries (Fig. 29.4). However, in practice, CO2 imaging is degraded by fragmentation of the CO2 bolus and patient motion related to discomfort caused by the CO2 injection. It also has poorer spatial resolution, which may impair subsequent endovascular treatment.29
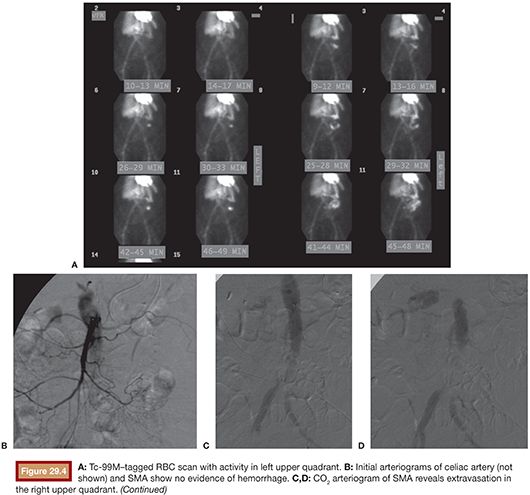
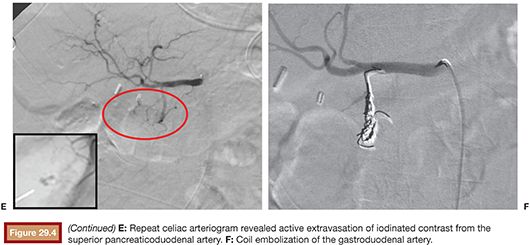
In one study, a negative bleeding focus was noted in 52% of cases, with a lower incidence in UGIB (46%) compared with lower GI bleeding (66%).30 Failure to localize a bleeding source may be attributed to slow or intermittent nature of the hemorrhage. In such cases, provocative angiography can aid in detection. Several techniques have been reported, including the administration of anticoagulants, vasodilators, and fibrinolytics, to temporarily augment bleeding and increase diagnostic sensitivity. However, this is rarely, if ever, used for UGIB because, unlike lower GI bleeding, angiography for UGIB is nearly always preceded by endoscopy, which commonly elucidates the source of bleeding. Moreover, the feasibility of empiric embolization in the upper GI tract does not justify the risks of provocative angiography.
If no arterial abnormality is seen, empiric embolization of the vessels supplying the area of concern can be performed. Empiric embolization is performed in 46% of endovascular cases of UGIB.28 This technique is low risk due to the rich collateral circulation of the upper GI tract. The two arteries targeted for empiric embolization are the left gastric artery and the GDA. The left gastric artery, which runs along the lesser curve of the stomach, supplies the distal esophagus, cardia, fundus, and incisura. There is collateralization with branches of the short gastric and right gastric arteries, which typically arise from the splenic and hepatic arteries respectively. The GDA supplies the remainder of the stomach and duodenum through the right gastroepiploic artery and branches of the pancreaticoduodenal arcade. There is collateralization with the left gastroepiploic artery, which arises from the distal splenic artery, and branches from the SMA. The SMA provides duodenal supply via the pancreaticoduodenal arcades28 (Table 29.4). There has been shown to be no statistical difference in outcomes between patients treated with empiric embolization versus embolization after angiographically demonstrated contrast extravasation.26,31,32 An alternative to empiric embolization in cases of negative angiography is to target branches supplying the area of endoscopically placed clips.
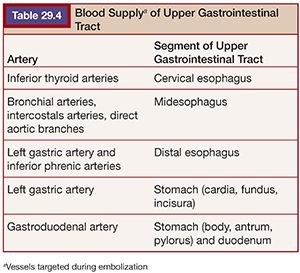
Although the number of arteries embolized may not impact clinical success of embolotherapy,25 it may affect subsequent surgical therapy. For example, embolization of both the left gastric and gastroduodenal arteries for treatment of a large gastric ulcer may impair attempts at subsequent partial gastrectomy in favor of total gastrectomy (Fig. 29.5).
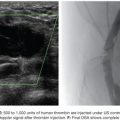
Superselection of vessels may be necessary to identify bleeding. This typically requires coaxial placement of a 3-Fr microcatheter through a 5-Fr catheter. At our institution, we commonly employ a Renegade (Boston Scientific Corporation, Natick, Massachusetts) or a Progreat (Terumo, Tokyo, Japan) microcatheter. The Renegade microcatheter is available in two types: HI-FLO and STC. The Renegade HI-FLO has a larger diameter (0.027 in) and is best suited for cases where particulate agents are used. The slightly smaller diameter (0.021 in) of the Renegade STC is preferred for the deployment of microcoils as the narrower lumen helps guard against intracatheter coil “formation,” particularly when using detachable coils. Particles can be administered through the Renegade STC with the caveat that the smaller diameter can lead to aggregation of particles and occlusion of the catheter. In such cases, the catheter can be carefully flushed with a 1-mL saline-filled syringe. The Progreat microcatheter is also available in various sizes, including 2.8-Fr (0.027 in) and 2.4-Fr (0.022 in). The former is available to order as a coaxial system that comes preloaded with a hydrophilic microwire.
Selecting the Gastroduodenal Artery
The GDA most commonly arises from the common hepatic artery. Less common variants include branches off the right hepatic artery or directly off the celiac axis.33 In many cases, the GDA is accessible with a 5-Fr catheter. The catheter is advanced from the celiac artery ostium into the proper hepatic artery over a Glidewire (Terumo, Tokyo, Japan). Care should be taken to avoid arterial dissection when using a Glidewire; this is especially true for patients with surgically altered anatomy (e.g., liver transplantation). Rotating the reverse curve catheter counterclockwise as it is being advanced can help when negotiating a tortuous common hepatic artery. Once in the proper hepatic artery, the catheter is carefully withdrawn until the tip engages the GDA.
Selecting the Left Gastric Artery
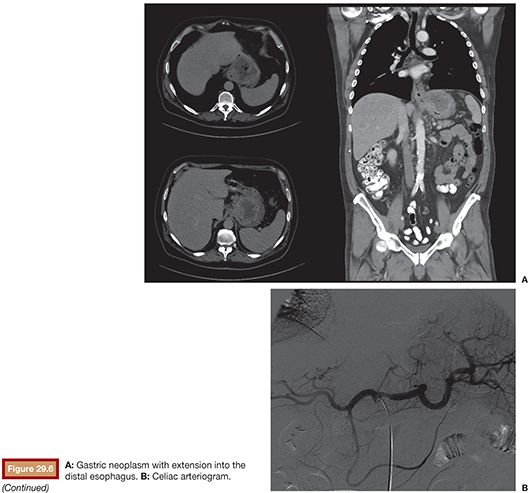
Normal celiac anatomy, with the left gastric artery being the smallest of the three primary branches of the hepatogastrosplenic trunk, is seen in 89% of patients. Less common variants include direct aortic origin (4.4%) and separate gastrosplenic and hepaticomesenteric trunks (2.6%).34 The left gastric artery courses cranially in a direction counter to the orientation of the celiac trunk. This feature can make it particularly challenging to catheterize. We favor accessing the celiac trunk with a VS1 catheter. The catheter is then carefully withdrawn until the tip is directed cranially and engages the origin of the left gastric artery. A microwire and microcatheter are then advanced into the vessel (Fig. 29.6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree