Fig. 1
(a) Renal pelvic transitional cell carcinoma (TCC) (arrow) appearing as a central soft-tissue mass in the echogenic renal sinus at US. (b) The same case examined by intravenous excretory urography revealing multiple filling defects (arrows) on renal pelvis
2.1.2 Intravenous Excretory Urography
Intravenous excretory urography was traditionally considered the first imaging modality to identify upper urinary tract tumors. Intravenous excretory urography presents extremely high accuracy in the detection of upper urinary tract tumors, even though it cannot define the extraluminal extension of the tumor. Lowe and Roylance (1976) described five distinct patterns of upper urinary tract malignancies at intravenous excretory urography, which could be translated for CT urography: (a) single (Fig. 2) or multiple (Fig. 3) discrete filling defects (35 %), (b) filling defects within distended calyces (26 %) (Fig. 4), (c) calyceal obliteration (calyceal amputation) (19 %) (Fig. 5), (d) hydronephrosis with renal enlargement caused by tumor obstruction of ureteropelvic junction (6 %) (Fig. 6), and (e) reduced renal function – excluded kidney – without renal enlargement caused by long-standing tumor obstruction of the ureteropelvic junction and atrophy (13 %) (Fig. 7).
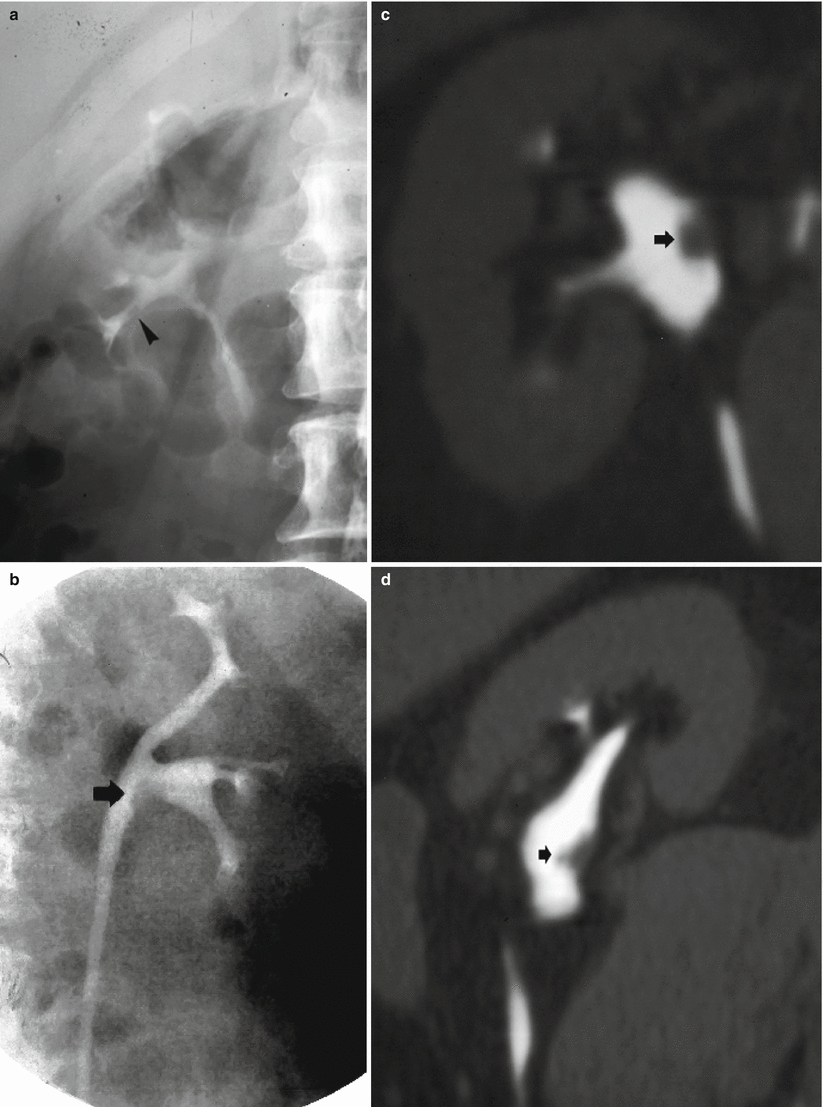
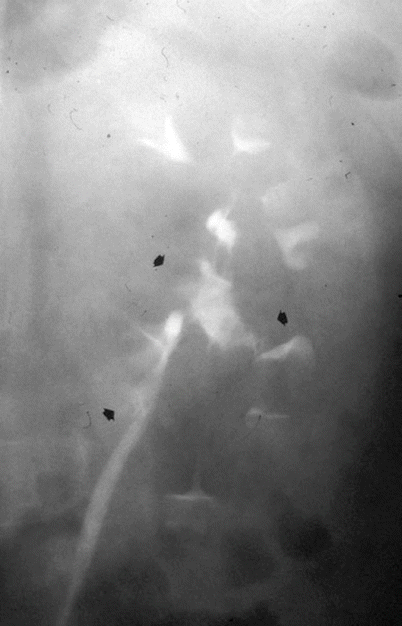
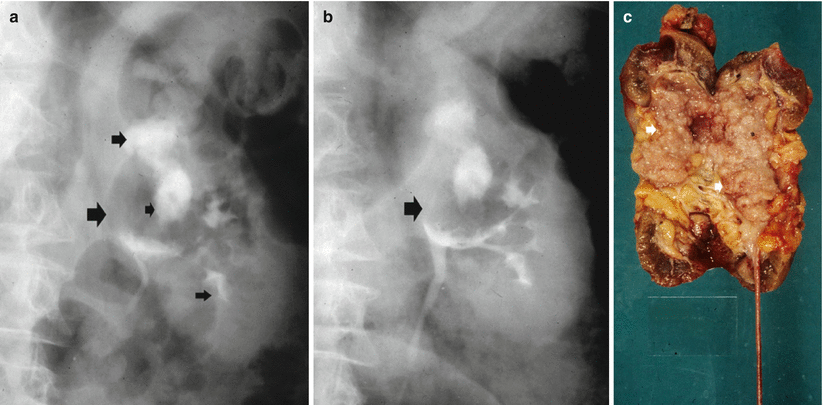
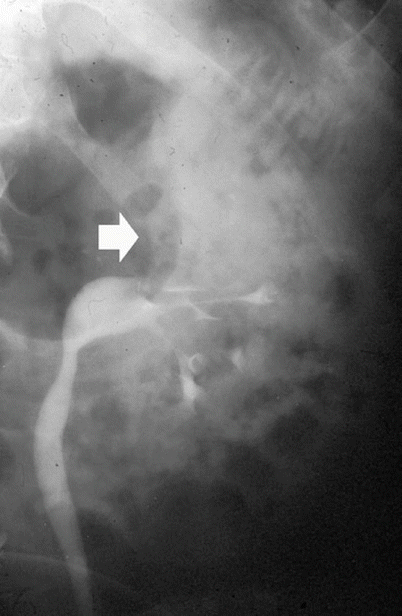
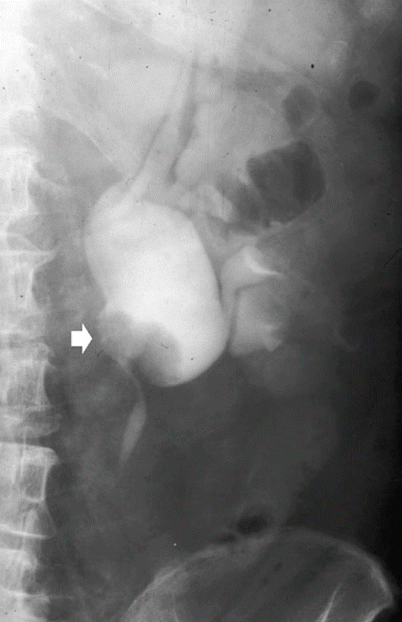
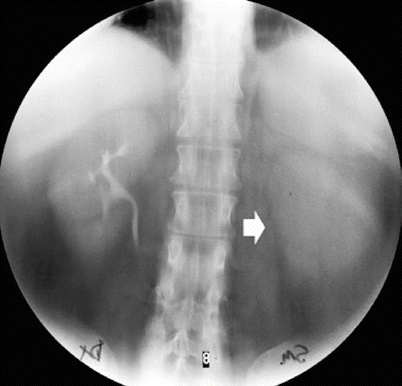

Fig. 2
(a–d) The different morphologic patterns of urinary tract malignancies. A single filling defect at excretory urography (a, b) and CT urography (c, d). The filling defect may involve a calyx, the infundibulum (arrowhead) (a), or the renal pelvis (arrow) (b–d). The CT urography with maximum intensity projection (MIP) 3D images allows a better definition of the relationship between the urothelial tumor (arrow) and the renal pelvis with the possibility of multiple views
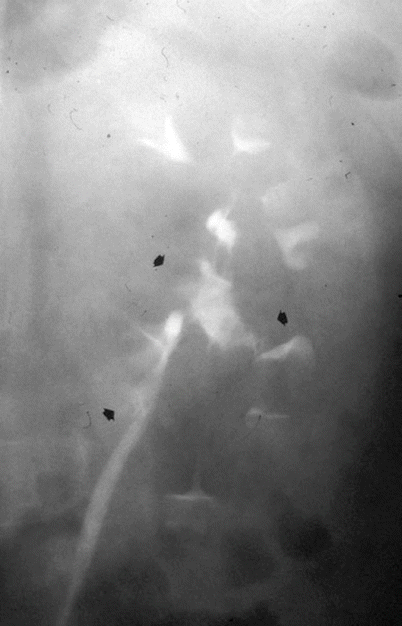
Fig. 3
The different morphologic patterns of urinary tract malignancies. Multiple discrete filling defects (arrows) at intravenous excretory urography

Fig. 4
(a–c) The different morphologic patterns of urinary tract malignancies. Multiple urothelial tumors (small arrows) within calyx dilatation at excretory urography (a, b). The surgical specimen (c) confirms the presence of multiple urothelial tumors

Fig. 5
The different morphologic patterns of urinary tract malignancies. Calyceal obliteration (calyceal amputation – arrow) at intravenous excretory urography

Fig. 6
The different morphologic patterns of urinary tract malignancies. Hydronephrosis with renal enlargement caused by tumor obstruction of ureteropelvic junction (arrow) at intravenous excretory urography

Fig. 7
The different morphologic patterns of urinary tract malignancies. Functionally excluded kidney (arrow) without renal enlargement caused by long-standing urothelial tumor obstruction of the urinary tract
Ureteric TCC classically appears as a solitary, polypoid filling defect with ureteric dilatation proximal to the lesion. The ureter itself may occasionally be fixed by diffuse ureteric wall infiltration from an intramural lesion. An “apple core” appearance may be observed with eccentric or encircling ureteric lesions. Malignant ureteric strictures may be circumferential or eccentric and can occasionally be confused with benign strictures, although ureteric fixation and nontapering margins are suggestive of malignancy.
2.1.3 Anterograde and Retrograde Pyelography
Intravenous excretory CT urography is the primary examination for the evaluation of upper urinary tract neoplasms. However, if complete obstruction exists and the kidney is nonfunctioning, anterograde or retrograde pyelography or static-fluid MR urography (see chapter “Magnetic resonance imaging of the kidney”) is necessary. Nowadays, both anterograde and retrograde direct pyelography are rarely performed.
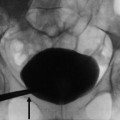
Anterograde pyelography may be performed during percutaneous needle pyelography in a nonfunctioning hydronephrotic kidney due to a TCC of the upper urinary tract. The upper margins of the obstructing tumor are outlined by contrast (Fig. 8), while the inferior extension can be depicted only by retrograde pyelography. Anterograde pyelography presents the risk of potential tumor seeding in the needle tract (Huang et al. 1995; Vikram et al. 2009), even though the probability of extraluminal seeding by percutaneous manipulation is thought to be very small. Anterograde pyelography is generally used only if retrograde cannulation of the ureteral orifice cannot be performed or if a percutaneous nephrostomy has been placed.
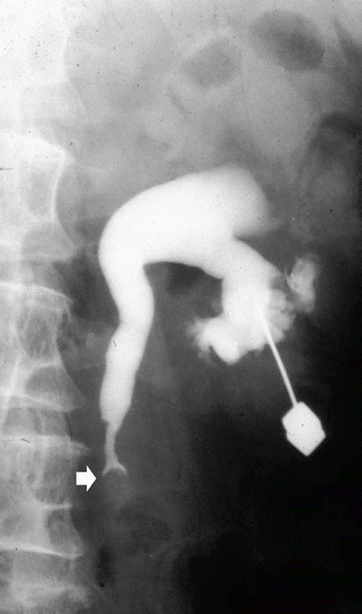

Fig. 8
Anterograde pyelography. The upper margins of the obstructing tumor (arrow) are outlined by contrast
Retrograde pyelography may be performed during cystoscopy or to further characterize abnormalities detected at intravenous excretory urography or multidetector CT urography, in inadequately excreting kidneys (O’Connor et al. 2008) or in cases of contrast material allergy. Although invasive, this procedure offers confirmation of the diagnosis by allowing collection of a sample for cytologic examination of localized urine collections. The findings suggestive of TCC, however, are similar to those seen on intravenous excretory urography (Vikram et al. 2009). As with intravenous excretory urography, on retrograde pyelography, renal TCC typically appears as an intraluminal filling defect, which may be smooth, irregular, or stippled. Opacification of a tumor-involved calix may show irregular papillary or nodular mucosa. If TCC involves an infundibulum, an “amputated” calix may be seen with or without focal hydronephrosis and calculi secondary to urinary stasis. Tumor-filled, distended calices are known as “oncocalices.” Retrograde pyelography allows confirmation of the radiologic diagnosis also facilitating ureteroscopy with biopsy or brushing and cytologic examination of localized urine collections. Anyway, it represents an invasive technique and it has been now almost completely replaced by CT urography.
An additional indicator, the goblet sign (Bergman sign) is seen on retrograde pyelography when dilatation distal to the tumor is seen in addition to the filling defect caused by the lesion itself (Daniels 1999). The goblet sign corresponds to the cup-shaped collection of contrast material distal to the ureteral filling defect, occurs due to slow tumor growth with resultant lumen expansion, and is not characteristic of more acute causes of obstruction. The goblet sign is a clue that the ureteral filling defect is a mass rather than a calculus (Daniels 1999). The slow expansion of a polypoid intraluminal tumoral mass from an uroepithelial carcinoma causes dilatation of the ureter distal as well as proximal to the mass. Propulsion of the mass distally during ureteral peristalsis further contributes to the dilatation of the ureter distal to the tumor and thus causes the cup-shaped collection of contrast material (Daniels 1999). On the other hand, the ureteral lumen just distal to a mechanical obstruction caused by a calculus will have a narrowed appearance due to wall spasm, edema, or both. Dilatation proximal to either a tumor or calculus varies with the degree of obstruction.
2.1.4 Multidetector CT Urography
The upper urinary tract, including the intrarenal urinary tract, has been traditionally evaluated with excretory urography which presents a limited sensitivity due to the suboptimal contrast resolution. Intravenous excretory urography was used for a large variety of clinical indications, and its utilization decreased when each of these indications was shown not to be clinically valid; cross-sectional imaging techniques have since proved superior to intravenous urography for most, if not all, remaining indications (Silverman et al. 2009). Even until the late 1990s, intravenous excretory urography was still considered the examination of choice for evaluating the urothelium (Silverman et al. 2009) since CT had a sensitivity of 50–68 % for the detection of upper urinary tract neoplasms (McCoy et al. 1991; Scolieri et al. 2000). When unenhanced CT was shown to reliably detect urolithiasis, intravenous urography was pronounced dead in 1999 with the caveat that there might remain rare instances in which it is an appropriate examination (Amis 1999). These instances included untangling complicated congenital anomalies, depicting surgical reconstructions of the urinary tract, and surveillance of patients with a history of urothelial carcinoma.
Nowadays, the development of CT urography (CTU) and MR urography has completely covered all the clinical indications previously addressed by excretory urography. CT urography, with the use of multidetector CT (MDCT) allowing the acquisition of thinner axial images and an isotropic volumetric dataset, provided a comprehensive examination of the kidneys, collecting systems, ureters, and bladder. Newer 16- and 64-row multidetector CT scanners offer a thinner collimation (as thin as 0.4 mm) with an improved z-axis spatial resolution allowing diagnostic reformatted images in any plane and faster imaging times which decrease motion-related artifacts. Some recent reports (Vrtiska et al. 2009) have shown that 64-MDCT technology with isotropic submillimeter spatial resolution allows coronal spatial resolution identical to that of traditional excretory urography using computed radiography (CR) detectors and that the 64-MDCT scanner is able to detect the smallest filling defects (0.25 mm) in a phantom model, which closely approximated the smallest filling defects that have been traditionally detected by excretory urography, including pyeloureteritis cystica and TCC. Moreover, CTU permits staging and assessment of the upper urinary tract in a single examination. CTU images should be evaluated with the correct CT window which corresponds to the bone window (as for the detection of renal stones on unenhanced CT images).
CTU is currently considered the most sensitive and comprehensive imaging modality for the evaluation of the entire urinary tract (Caoili et al. 2002. The indications for CT are now expanded to include hematuria (Joffe et al. 2003; Lang et al. 2004; O’Malley et al. 2003), and CT urography has essentially replaced excretory urography in this clinical setting (Heneghan et al. 2001; McNicholas et al. 1998; McTavish et al. 2002; Noroozian et al. 2004). In general, MDCT urography can be tailored toward the clinical question based on clinical information. For benign indications where only the excretory phase will be relevant (variant urinary tract anatomy, ureteral pseudodiverticulosis, and iatrogenic ureter trauma), single-phase MDCT urography can suffice. Patients with more complex benign diseases and those with chronic symptomatic urolithiasis (complex infections, percutaneous nephrostolithotomy planning) may benefit from adding an unenhanced phase to the excretory phase. In chronic urolithiasis without complete obstruction, furosemide-assisted CT urography can demonstrate most ureteral stones within the enhanced urine. So for the evaluation of hydronephrosis due to obstruction by stones, the unenhanced phase may be safely deleted, whereas diagnosis of small nonobstructing stones may be done by an unenhanced phase limited to the kidneys.
It is difficult to obtain a single image of all urinary collecting system segments in an opacified and distended state, and intravenous saline (250 mL) or furosemide injection improves the urinary tract and ureteral opacification and distension. It is not just about depicting anatomy, since opacification and distension help in the detection of small urothelial neoplasms. In addition to the evaluation of hematuria, MDCT urography can be useful in the surveillance of patients with suspected urothelial cancer (positive urine cytology), follow-up of urothelial cancers (Silverman et al. 2009), patients with obstructive uropathy (e.g., hydronephrosis, hydroureter of unknown etiology), or any time, a comprehensive evaluation of the urinary tract is warranted. MDCT urography can detect many different urinary tract abnormalities including tiny uroepithelial neoplasms (Caoili et al. 2002, 2005; Chow et al. 2007) with a sensitivity of 90–97 % (Fritz et al. 2006; Cowan et al. 2007), even greater than on retrograde pyelography (McCarthy and Cowan 2002), with the possibility to employ coronal, sagittal, and curved-planar reformatted images and 3D reconstructed images (Tsili et al. 2007; Dillman et al. 2008) in addition to axial reconstructed images.
On MDCT urography, the majority (55–60 %) of renal TCCs present as wall thickening with a broad base and frondlike morphologic structure and manifest as sessile filling defects within the contrast-enhanced collecting system in the excretory phase. MDCT urography presents a sensitivity of 86–89 % in detecting lesions with these features (Caoili et al. 2005) with high positive and negative predictive values. Postprocessing techniques including curved multiplanar reformations (MPRs) and 3D volume rendering (VR) are useful in urothelial tumor detection, while maximum intensity projection (MIP) 3D images do not detect urothelial tumor since they are less dense than contrast medium. Filling defects may be single (Figs. 2 and 9) or multiple (Fig. 10) and smooth, irregular (Fig. 11), or stippled and tend to expand centrifugally with compression of the renal sinus fat (Browne et al. 2005) (Fig. 12). These lesions present as central mass within the renal pelvis (Urban et al. 1997). The stipple sign (Fig. 13) refers to tracking of contrast material into the interstices of a papillary lesion. However, this sign may also be seen with blood clot and fungus balls and should be interpreted with caution. Stricture-like lesions of the pelvicalyceal system may be evident and, if multiple, may mimic renal tuberculosis. Filling defects within dilated calices may occur secondary to tumor obstruction of the infundibulum and may lead to calyceal “amputation.” If calices fail to opacify with contrast material, they are known as “phantom calices.” Other appearances include pelvicalyceal irregularity, oncocalyx (Fig. 14) (tumor-filled, distended calices), and focally obstructed calices. Early tumors confined to the muscularis are separated from the renal parenchyma by renal sinus fat or excreted contrast material and have normal-appearing peripelvic fat. Advanced TCC extends into the renal parenchyma in an infiltrating pattern that distorts normal architecture (Fig. 15). However, reniform shape is typically preserved (Fig. 16), unlike in renal cell carcinoma. About 15 % of renal TCCs have a more aggressive growth pattern, and they will appear as an infiltrative process in the renal parenchyma (Fig. 17), often with obliteration of the source collecting system structure (Baron et al. 1982), or as a focal or diffuse circumferential wall thickening (Fig. 18) (Caoili et al. 2005). Diffusely infiltrating TCC is characterized by thickening and induration of the wall of the renal pelvis or ureter (Figs. 18 and 19). Wall thickening of renal pelvis or ureter, or tumor extension into the renal pelvis presents as a hypodense filling defect that stands out against the contrast media within the lumen of the urinary tract.
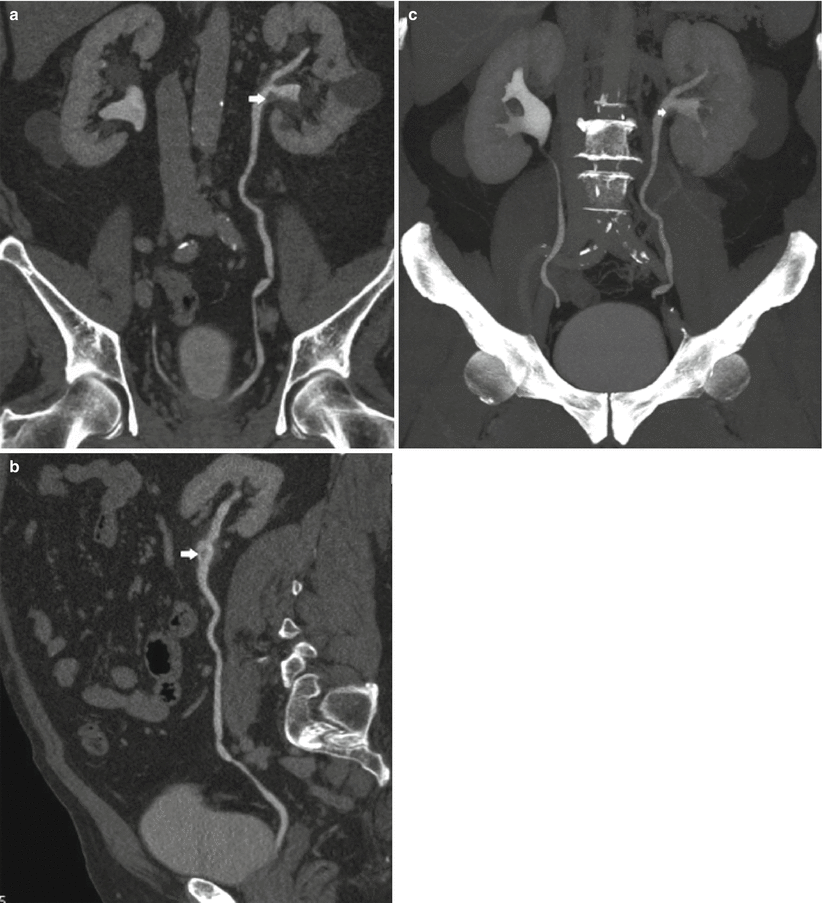
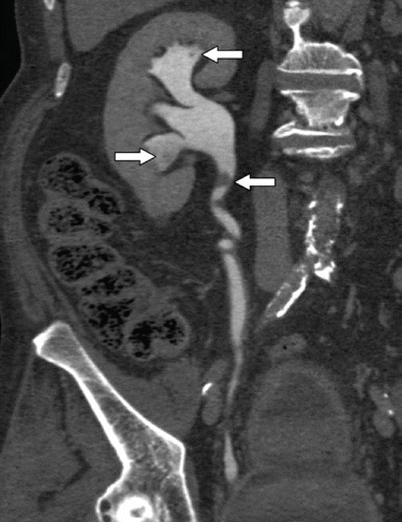
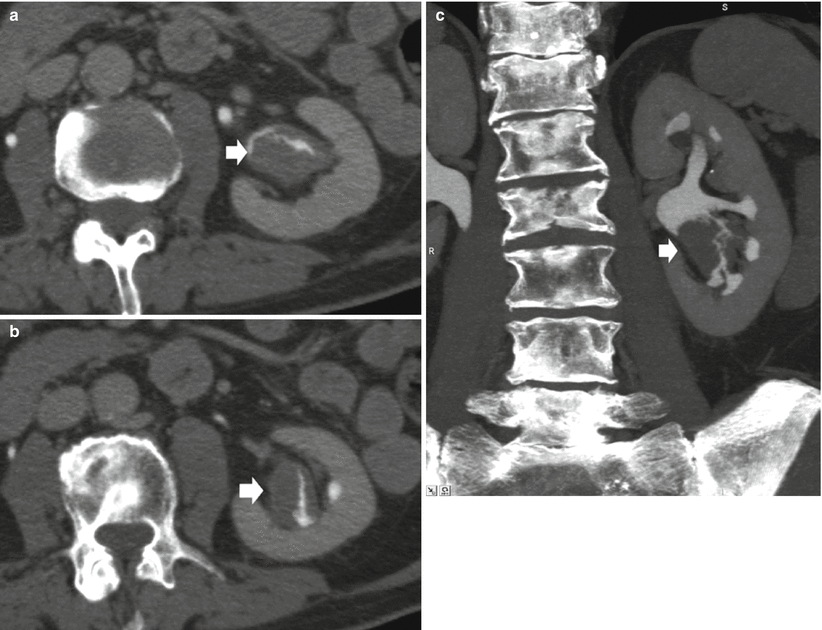
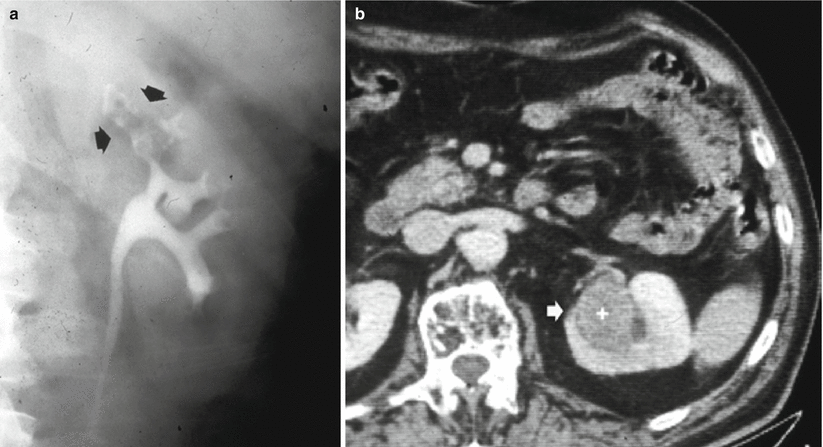
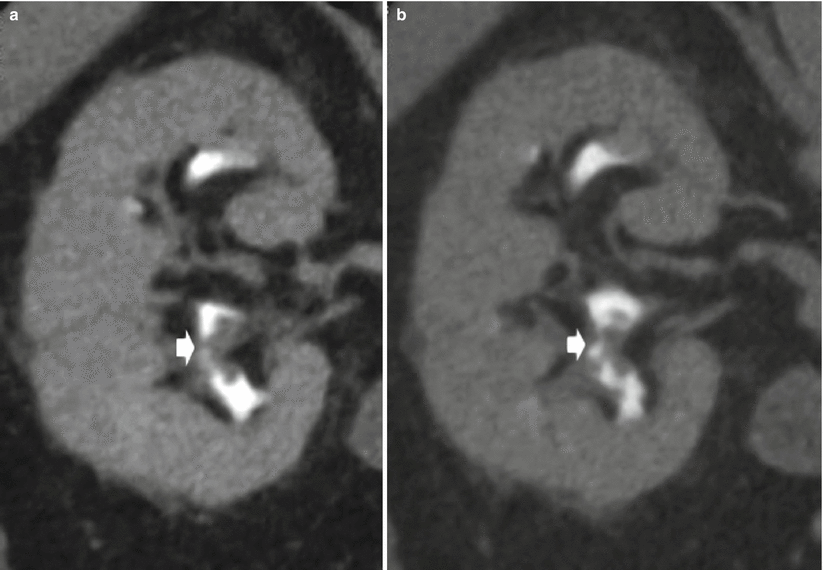
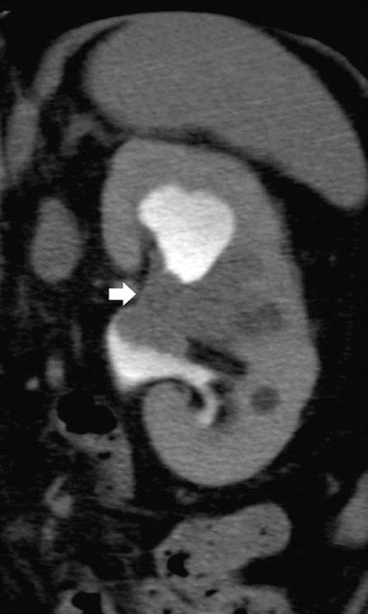
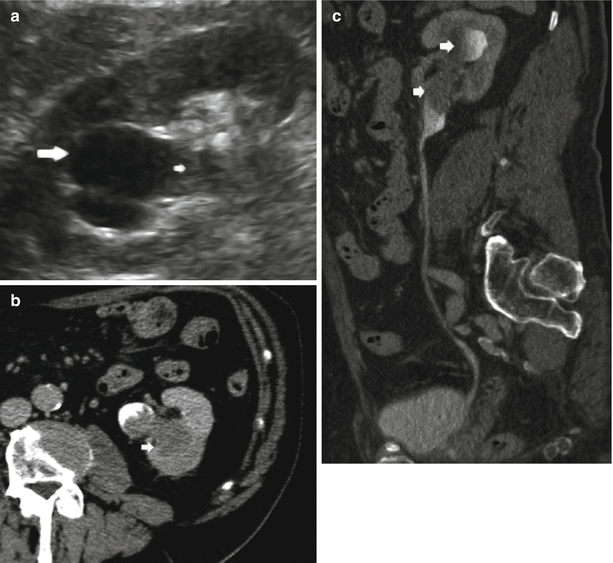
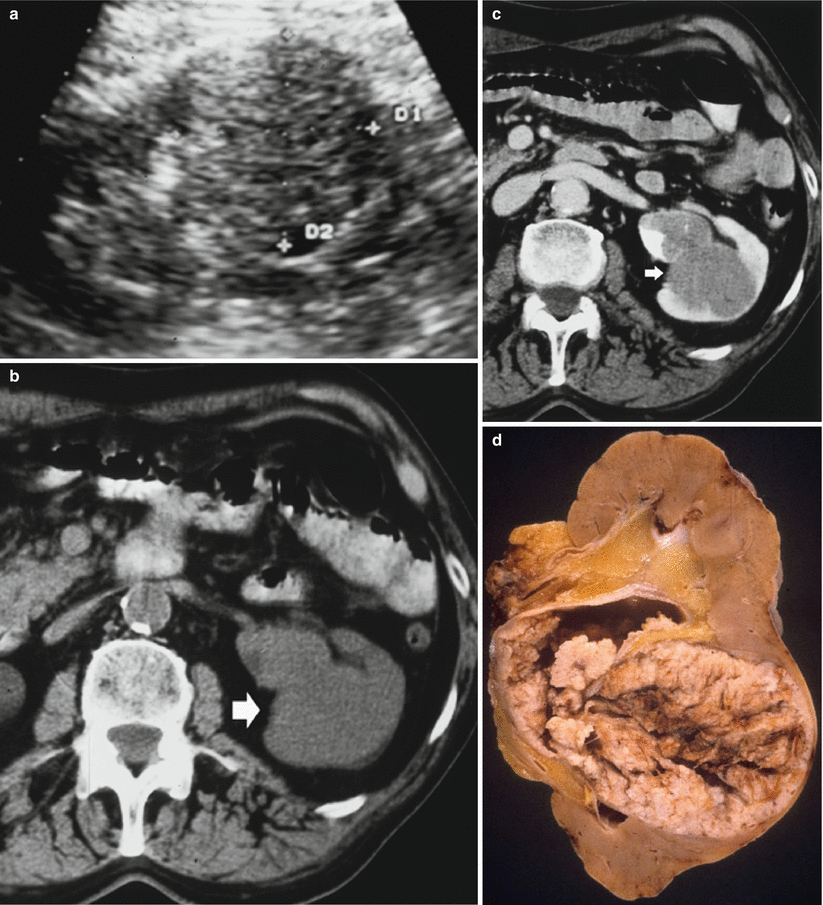
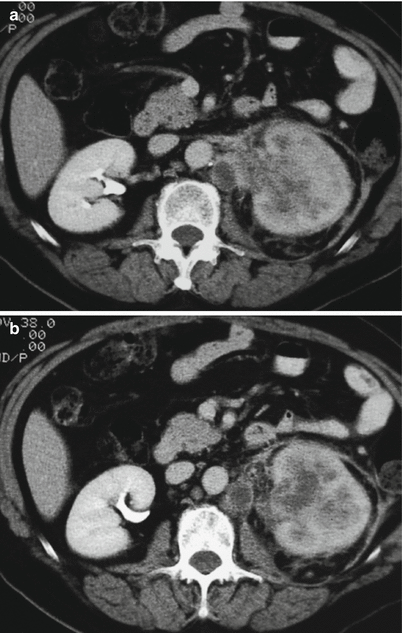
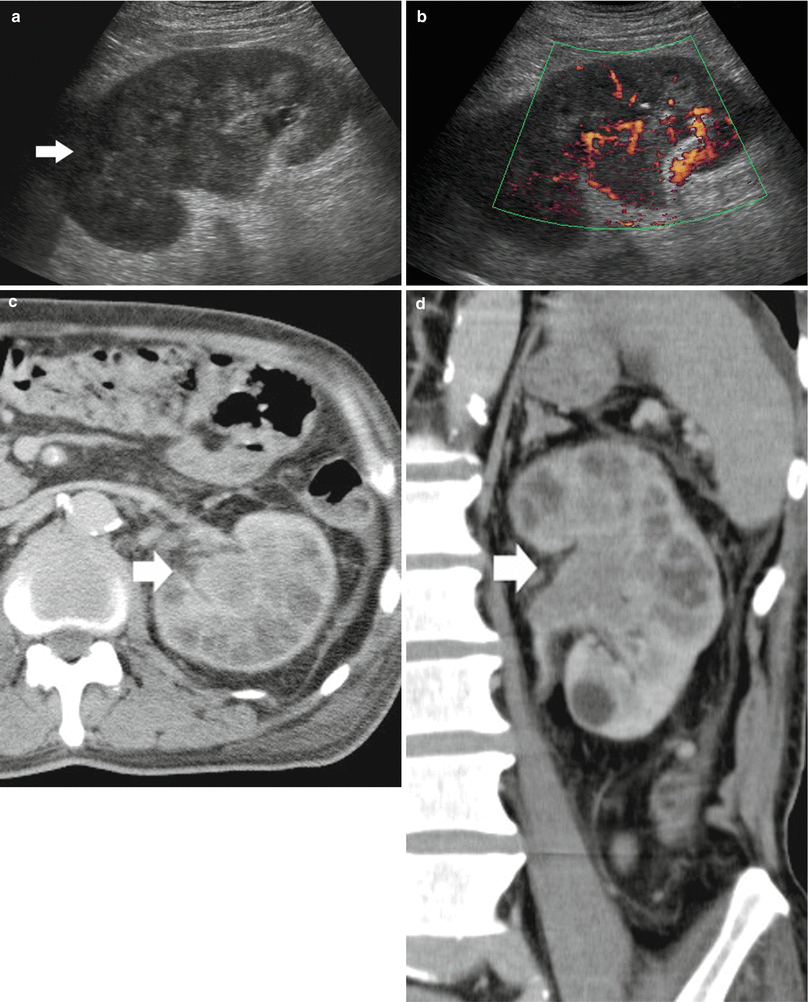
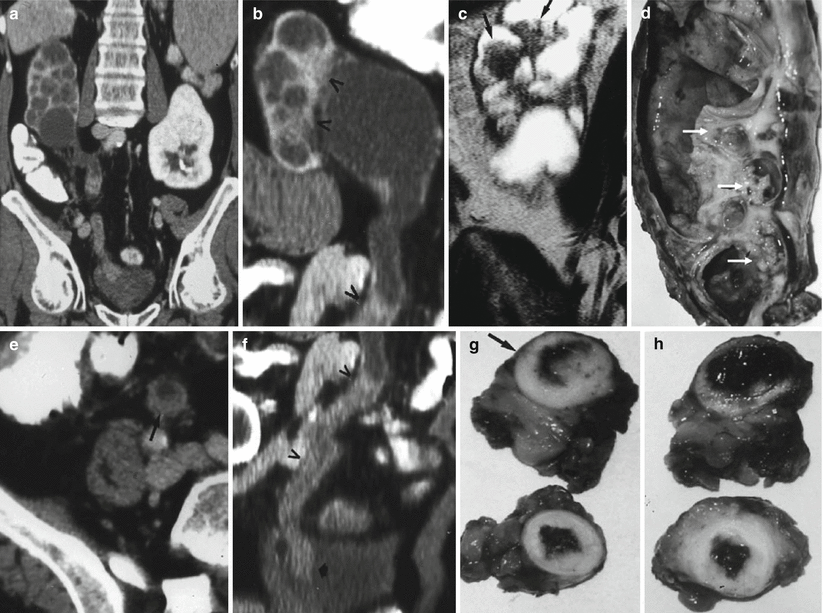

Fig. 9
(a–c) Seventy-five-year-old male patient with persistent hematuria. Single TCC (arrow) at CT urography (a), curved-planar reformations (b), and volume rendering image (c). Small TCC (arrow) within the left renal collecting system with no sign of infiltration of the adjacent renal parenchyma

Fig. 10
CT urography. Multiple filling defects (arrows) corresponding to TCC without infiltration of the adjacent renal parenchyma

Fig. 11
(a–c) TCC without infiltration of the adjacent renal parenchyma. CT urography (a, b) transverse plane and (c) reformations on the coronal plane. The urothelial carcinoma (arrow) determines a stricture-like lesion of the pelvicalyceal system with stenosis of the infundibulum and calcyceal obliteration and slight compression of the renal sinus fat

Fig. 12
(a, b) Single TCC without infiltration of the adjacent renal parenchyma. (a) Excretory urography, (b) contrast-enhanced CT excretory phase. (a) Multiple filling defects (arrows) on the intrarenal urinary tract with calyceal obliteration. (b) The urothelial carcinoma (arrow) presents a close proximity with the renal parenchyma without signs of parenchymal infiltration but with evident compression of the renal sinus fat

Fig. 13
(a, b) CT urography. TCC of the right kidney. The filling defect (arrow) presents the stipple sign referring to tracking of contrast material into the interstices of a papillary lesion

Fig. 14
Multidetector CT (MDCT) urography. Filling defects (arrow) with dilatation of the upper calyces secondary to tumor obstruction of the infundibulum, and tumor-filled, distended calyces of the mesorenal region (oncocalyces)

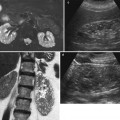
Fig. 15
(a–c) Eighty-four-year-old woman presenting with hematuria. (a) Ultrasound. A renal mass (small arrow) is identified on the left kidney within the renal urinary tract with segmental dilatation of the upper urinary tract (large arrow). Contrast-enhanced CT during nephrographic phase (b). A hypodense mass (arrow) infiltrating the perirenal fat and renal parenchyma is visualized with dilatation of the intrarenal urinary tract. (c) Contrast-enhanced CT during nephrographic phase, curved reformations (c). A hypodense mass (arrow) is confirmed within the dilated intrarenal urinary tract with evidence of tumor-filled, distended calices (oncocalyces). Single TCC was diagnosed at histologic analysis

Fig. 16
(a–d) Eighty-year-old woman presenting with hematuria. (a) Ultrasound. A renal mass is identified on the left kidney. Unenhanced (b) and contrast-enhanced CT during nephrographic phase (c). A hypodense mass (arrow) infiltrating the renal sinus fat and renal parenchyma is visualized. (d) Gross surgical specimen. Single TCC was diagnosed at histologic analysis

Fig. 17
(a, b) TCC at CT urography with diffuse infiltration of the whole right kidney. Contrast-enhanced CT, excretory phase. Diffuse infiltration of the renal parenchyma in the left kidney by a TCC. No contrast excretion is evident of the whole left kidney which is functionally excluded

Fig. 18
(a–d) TCC at CT urography with diffuse infiltration of the whole right kidney. (a, b) Ultrasound and power Doppler ultrasound. Solid tissue infiltrating the whole kidney (arrow). (c, d) Contrast-enhanced CT, excretory phase, transverse and coronal reformation. Diffuse infiltration of the renal parenchyma in the left kidney by a transitional cell carcinoma originating from renal pelvis (arrow). No contrast excretion is evident on the left kidney which is functionally excluded

Fig. 19
(a–h) Multicentric diffusely infiltrating TCC involving both the intra- and extrarenal collecting system. (a) Contrast-enhanced CT.Coronal reformation. Dilatation of the right intrarenal urinary tract with evidence of diffuse ureteral involvement by the tumor (b) Contrast-enhanced CT. Coronal reformation. Dilatation of the right intrarenal urinary tract with diffuse renal pelvis and ureteral infiltration (arrowheads). (c) T2-weighted turbo spin echo MR sequence. Coronal plane. Multiple mural nodules (arrows) are evident on the dilated intrarenal urinary tract. (d) Gross surgical specimen. Multiple mural tumors on the renal pelvis (arrows) (e, f) Contrast-enhanced CT. Transverse plane (e) and coronal reformation (f). Diffuse ureteral infiltration by the TCC (arrow in e, and arrowheads in f). (g, h) Gross surgical specimen of the ureteral carcinoma with macroscopic whitish appearance of the tumor (arrow)
Ureteric TCC is typically seen as single or multiple ureteric filling defects with or without surface stippling and proximal ureteric dilatation (Fig. 20). It is important to remember that long-standing tumor obstruction of the ureteropelvic junction or ureter may lead to generalized hydronephrosis and poor excretion. This is a major disadvantage of intravenous excretory urography when compared with CT urography, which allows the assessment of nonfunctioning kidneys. Upper tract filling defects may be nonspecific at intravenous excretory urography, and obstruction of pelvicalyceal drainage may obscure distal synchronous ureteric tumors, meaning that retrograde pyelography is usually performed to further assess these patients.
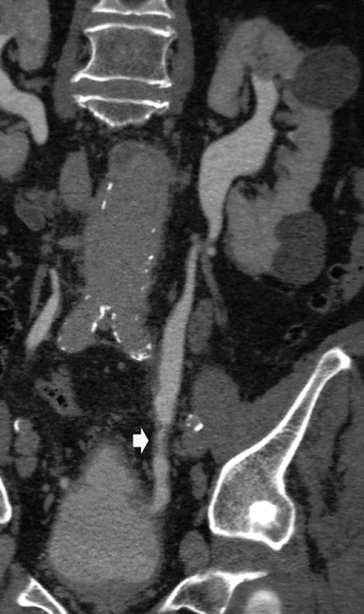

Fig. 20
TCC of the distal ureter. CT urography. Curved-planar reformat. An irregular thickening of the distal ureteral wall (arrow) is evident during the excretory phase. Some cystic lesions are also evident in the left renal parenchyma
2.1.5 CT
Contrast-enhanced CT is well established in the preoperative staging and assessment of upper tract TCC (Urban et al. 1997




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree