Anatomy, embryology, pathophysiology
- ◼
The urothelium is the mucosa composed of transitional epithelium that lines the renal calyces and pelvis, ureters, bladder, and much of the urethra. Further anatomic and clinical classification defines the upper tract as the renal collecting system and ureter, whereas the lower tract pertains to the bladder and urethra.
- ◼
The ureters connect the renal collecting system to the bladder. The average ureteral length in the adult patient is approximately 30 cm. This is a retroperitoneal structure that courses just over the medial aspect of the psoas muscles and anterior to the common/external iliac artery. The ureter then courses along the lateral pelvic wall before turning medially toward the bladder. In females, the ureter extends beneath the broad ligament and uterine artery, whereas in males, the ureter courses under the vas deferens. Upon joining the bladder, the ureters course submucosally within the bladder wall for 2 to 3 cm before terminating at the ureteral orifice.
- ◼
The intramural course of the distal ureter helps prevent urinary reflux. This results in a physiological narrowing at the ureterovesical junction, which should not be confused with pathological stricture.
- ◼
- ◼
The bladder is a distensible hollow organ acting as a reservoir for urine with the adult bladder having a normal capacity of 400 to 500 mL. The detrusor muscle forms the muscularis propria and is composed of interweaving smooth-muscle bundles. The internal sphincter is a thickening of the detrusor muscle at the bladder neck, which extends distally into the proximal urethra. The region between the ureteral orifices and the bladder neck is defined as the trigone. The bladder is tethered to the umbilicus by the median umbilical ligament (obliterated urachus). In females, the bladder is anterior to the uterus and vagina whereas in males, it sits anterior to the seminal vesicles and rectum.
- ◼
The male urethra is anatomically divided into the posterior and anterior portions. The posterior urethra includes the prostatic and membranous segments that extend through the urogenital diaphragm. Beyond this level is the anterior urethra, which is composed of the bulbous and penile urethra. The urothelium forms the mucosal layer of the urethra to the level of the penile glans, where there is a transition to squamous cell epithelium. The female urethra is about 3 to 4 times shorter and extends from the bladder neck to beneath the pubic symphysis and terminates just anterior to the vagina. Urothelium lines the female urethra until its distal portion where there is also a transition to squamous epithelium.
- ◼
There is a wide range of processes that can cause urothelial narrowing and obstruction. These include benign as well as malignant etiologies, which are detailed later in the section titled “Specific Disease Processes”.
Techniques
The imaging appearance of ureteral strictures depends on the underlying etiology. Lesions may cause intrinsic narrowing of the ureter, whereas extrinsic processes may cause ureteral stricturing by encasement, compressive mass effect and infiltration.
- ◼
Filling defects in the ureter are classified as intraluminal, mucosal, or submucosal. These can often be distinguished by observing the relationship between the lesion and the ureteral wall as outlined by intraluminal ureteral contrast material. Submucosal lesions typically demonstrate obtuse angles with the wall; mucosal lesions demonstrate acute angles, and intraluminal lesions will be completely surrounded by contrast.
- ◼
Infiltrative processes will often show segmental ureteral narrowing with mucosal irregularity and circumferential mural thickening ( Fig. 27.1 ). Ureteral strictures caused by encasement may have a tapered appearance with preservation of smooth mucosal contour; however, abrupt caliber transition with upstream ureteral dilatation is an alternate appearance.
- ◼
“Bullet and bodkin” sign: descriptor for abrupt luminal narrowing on intravenous pyelography from encasement (which may be benign or malignant).

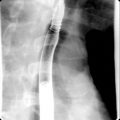
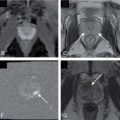
Fig. 27.1
48-year-old man who presented with gross hematuria. Excretory magnetic resonance urography images demonstrate long-segment diffuse right urothelial thickening with thin preserved ureteral lumen containing excreted contrast ( arrows ). Biopsy confirmed ureteral amyloidosis.
- ◼
- ◼
Strictures can range greatly in length and be isolated to a single ureter, bilateral, and even bilateral and multifocal.
Imaging techniques for urinary tract obstruction are detailed in the preceding Chapter 26 . A brief overview is provided here with attention to the ureters.
- ◼
Conventional radiography plays no significant role in evaluation of ureteral pathology. Techniques, such as excretory urography, have traditionally been helpful in evaluating ureteral course and caliber, as well as detecting focal filling defects and strictures, although have largely been replaced by computed tomography (CT). Retrograde urography is a technique usually performed by urologists as it requires cystoscopic guidance for ureteral canalization.
- ◼
Ultrasonography is a useful modality to evaluate for urinary tract obstruction but has limited use for imaging ureteral strictures.
- ◼
CT is the imaging workhorse for ureteral evaluation and allows for identifying and better characterizing strictures. The added benefit of CT is more complete assessment of the extent of ureteral involvement, as well as visualizing adjacent structures, which is helpful in differentiating intrinsic from extrinsic etiologies.
- ◼
Magnetic resonance urography (MRU) is gaining ground in evaluation of ureteral disease. Intrinsic T2 hyperintensity of urine allows for noncontrast evaluation of the urinary tract, especially if there is a dilated collecting system and gadolinium enhanced imaging provides even further advantages. Although MRU can be limited by artifacts, patient cooperation, and incomplete ureteral distention, the lack of ionizing radiation has made this study especially useful in the pediatric and pregnant populations.
- ◼
Nuclear medicine examinations are not typically used in evaluating ureteral disease. Positron emission tomography is of limited use because of normal excretion of fluorodeoxyglucose via the urinary tract.
Protocols
Computed tomography protocol considerations
- ◼
CT urography is a comprehensive multiphase examination that is commonly indicated for hematuria and evaluation of the complete urinary tract.
- ◼
Typical protocol includes three-phase examination with noncontrast, nephrographic and excretory phase acquisitions ( Fig. 27.2 ).

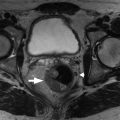
Fig. 27.2
63-year-old man with hematuria presents for computed tomography urography. Noncontrast axial image through the right renal lower pole (A) demonstrates soft-tissue density with focal calcification within the calyx. Nephrographic phase (B) shows lesion enhancement whereas the excretory phase (C) clearly outlines the calyceal filling defect. Biopsy consistent with transitional cell carcinoma.
- ◼
To decrease radiation exposure, many institutions have switched to the split-bolus injection technique, which allows for simultaneous nephrographic and excretory phase imaging.
- ◼
There is increasing prevalence of dual-energy CT, which allows for single acquisition using a split bolus with virtual reconstruction of noncontrast images, thus further reducing radiation dose.
- ◼
- ◼
Three-dimensional (3D) image reconstruction techniques, such as maximum intensity projection (MIP) and volume-rendered imaging have been shown to improve urothelial lesion detectability ( Fig. 27.3 ).

Fig. 27.3
A three-dimensional image reconstruction maximum intensity projection demonstrating bilateral ureters in a single plane.
Magnetic resonance protocol considerations
MR urography is traditionally performed with two techniques. For pregnant patients or patients with decreased renal function, images are acquired using static fluid MR urography, which requires no intravenous (IV) contrast. In patients who can receive IV gadolinium-based contrast, excretory MR urography is performed. In addition, regardless of the MR urography technique, standard multiplanar T1 and T2-weighted sequences are obtained to help delineate underlying pathology.
Static fluid magnetic resonance urography
- ◼
Analogous to MR cholangiopancreatography. Heavily T2-weighted sequences are obtained through the urinary tract with typical protocols, including breath-hold thick or thin-slab single-shot fast spin echo sequences and optional MR cine acquisition.
- ◼
Imaging relies on fluid within the urinary tract. In patients without dilated systems, prehydration with IV fluid may be beneficial.
- ◼
Added advantage in patients with nonfunctioning or severely obstructed urinary systems compared with excretory MR urography.
- ◼
Excretory magnetic resonance urography
- ◼
More comprehensive examination and allows for multiphase postcontrast imaging (e.g., early arterial, corticomedullary, nephrographic, and delayed phase). Delayed images through the collecting system can be acquired at higher spatial resolution than static fluid MR urography.
- ◼
Additional advantage over static fluid MR urography is improved lesion characterization.
- ◼
Low-dose gadolinium (~0.1 mmol/kg) is recommended, as well as consideration of diuretics, such as furosemide, to improve urinary contrast distribution and avoid T2-shortening effects, which can result in decreased urine signal.
- ◼
Specific disease processes
Urothelial disease has a broad range of etiologies, including congenital, inflammatory, infectious, malignant, traumatic, and idiopathic conditions ( Box 27.1 ).