Since their introduction, gadolinium-based contrast media are routinely used in most CNS MR imaging indications. Due to their paramagnetic effect, they significantly shorten the T1 relaxation times of the tissue and are therefore applied to improve the sensitivity and specificity of CNS diseases and to allow a better treatment decision, planning, and follow-up. More recently, contrast media have also been used to allow the measurement of tissue perfusion and to follow the time course of enhancement in dynamic contrast-enhanced imaging studies. Routinely they are used to enhance MR angiography studies.
- •
Gadolinium-based contrast media are routinely used in most CNS MR imaging indications, including assessment of CNS tumors, vascular pathologies, infections, degenerative diseases, and posttreatment imaging.
- •
Contrast media is applied to improve the sensitivity and specificity of CNS diseases and to allow a better treatment decision, planning, and follow-up.
- •
The standard dose used is 0.1 mmol/kg of body weight, with some exceptions that allow injection of up to a triple dose.
- •
More recently, contrast media have also been used to allow the measurement of tissue perfusion and to follow the time course of enhancement in dynamic contrast-enhanced imaging or dynamic MR angiography studies.
- •
With the presence of the BBB, contrast media does not leak into the tissue. Only vascular structures and areas of the brain that have no BBB (choroid plexus, pineal and anterior lobe of pituitary gland) physiologically enhance after contrast injection. The mechanisms of tissue enhancement in the brain are related to a higher vascularity of the pathology or a disruption of the BBB.
- •
Tissue enhancement is, besides the degree of BBB, disruption further dependent on the applied magnetic field strength, with higher field providing a better enhancement and the applied dose of contrast media.
Introduction
The goals and indications of central nervous (CNS) or neuroimaging are multiple and involve detection of pathologies, including making a precise diagnosis and a differential diagnosis, and detection of disease complications that require an immediate intervention and proved to be an essential part of the decision-making process for therapy. In the case of neoplastic disorders, for example, neuroimaging can precisely define the location and accurately delineate the lesion before intervention. In the case of radiation oncology, it should precisely define and demarcate the margins for targeted intervention. Neuroimaging is also mandatory after any therapeutic intervention for monitoring of disease and for detection and monitoring of possible side effects.
In emergency medicine, computed tomography (CT) assumes a critical role for the evaluation of traumatic and nontraumatic evaluations. CT scanners are fast and widely available, which makes CT a great choice for assessment of neurologic or neurosurgical emergencies. Usually, a conventional noncontrast brain CT is performed in an emergency to detect, for example, fractures and intracranial bleeding, and also to exclude tumors and subdural/extradural hematomas. Nevertheless, the intrinsic tissue contrast is limited in the CT images. Neuroimaging with CT relies, therefore, deeply on contrast media to improve lesion detection (sensitivity) and characterization (specificity). Furthermore, contrast agents can be used for functional assessment of physiologic process: the blood perfusion and for the depiction of the vessel compartment (angiography/venography). In CT angiography, the images are obtained a few seconds after high-flow contrast injection and most of the observed enhancement is intravascular. When CT acquisition is delayed for 10 to 15 minutes after contrast agent injection, most of the observed enhancement is interstitial. When CT imaging is done at intermediate times, the enhancement reflects a combination of both intravascular and interstitial components.
Because of its high tissue contrast and noninvasiveness, magnetic resonance (MR) imaging is accepted as the most sensitive method for diagnosing diseases of the CNS. MR imaging enables accurate recognition and determination of the dimensions of CNS pathologies and their surrounding tissue. This requires a high CNS-to-lesion contrast, which both depends on the signal intensity of the lesion relative to that of the surrounding normal tissue and the best description of the physiologic tissue and vasculature not affected. Furthermore, detailed information on the internal morphology of the lesion is essential for differential diagnosis, grading, and for the selection and planning of therapy.
Although MR imaging using standard nonenhanced T1-weighted and T2-weighted sequences has proven to be very sensitive in the detection of pathologic lesions, for most CNS diseases and for many of the currently available functional MR imaging methods, the use of MR contrast media is mandatory. The standard dose used for MR imaging of the CNS is 0.1 mmol/kg body weight, although numerous studies have shown that lesion detection may be improved with the use of higher doses and dedicated sequences. A higher dosage also allows for the combination of a number of advanced, mostly contrast-enhanced MR imaging techniques that have been developed that provide new insights into the pathophysiology of CNS diseases, as well as to better describe the angioarchitecture using contrast-enhanced MR angiography (CE-MRA). One of these techniques, perfusion MR imaging, is now recognized as an important new means for assessing tumor grading and follow-up of various treatment strategies or to better assess cerebrovascular diseases. Another of these techniques, dynamic contrast-enhanced MR imaging, is also gaining acceptance for the same purposes. In this article, the reader is provided with the fundamental features of the contrast mechanisms in MR imaging neuroimaging, the basic methodologies and the first clinical experience with the contrast-enhanced functional imaging tools, and some of the classical indications for contrast media. The different properties of the currently available contrast media and the dosage and field dependencies have been discussed in the article by Dr. Kanal, elsewhere in this issue.
In neuroimaging, pathologic enhancement can occur in several regions:
- 1.
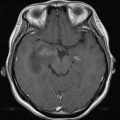
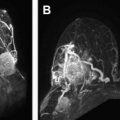
Abnormal enhancement within vessels without breakdown of the blood-brain-barrier (BBB), which reflects neovascularity, macrovasodilatation or microvasodilatation (aneurysm), and shortened transit time or shunting, including arteriovenous malformations ( Fig. 1 ).

Fig. 1
Fluid attenuated inversion recovery (FLAIR) ( A ), gradient-echo T2 ( B ), T1 spin echo (SE) ( C ), and contrast-enhanced T1 SE ( D ) in a patient with cerebral arteriovenous malformations. The malformation presents on FLAIR and T2 a signal drop, which is partially related to flow-void and partially attributable to microbleedings. The high signal on unenhanced T1 is attributable to blood, the marked contrast enhancement represents vascularization in the center of the malformation.
- 2.
Extra-axial lesions with no BBB, such as meningioma, acoustic schwannoma, or granulomatous disease.
- 3.
Breakdown of BBB with leakage of contrast, including neoplastic disease, infection, infarction, inflammation with demyelinating disease, and trauma.
Listing and definition of contrast media indications
Mechanism of Contrast Enhancement in CNS
Owing to the presence of the BBB, the currently available contrast media do not leak into the brain tissue. Only vascular structures and areas of the brain that have no BBB (choroid plexus, pineal and anterior lobe of pituitary gland) physiologically enhance after contrast injection. The BBB consists of a complex of capillary endothelial cells, pericytes, and astroglial and perivascular macrophages and serves as an effective physical barrier to the entry of lipophobic substances into the brain. The BBB blocks all molecules except those that cross cell membranes by means of lipid solubility (such as oxygen, carbon dioxide, ethanol, and steroid hormones) and those that are allowed in by specific transport systems (such as sugars and some amino acids). Substances with a molecular weight higher than 180 Da, which include all available imaging contrast media, generally cannot cross the BBB.
The integrity of the BBB can be altered by a variety of pathologic and physiologic circumstances that increase the permeability, both for contrast media and drug delivery. A disruption of the BBB may be caused by osmotic means (eg, steroids), biochemically by the use of vasoactive substances, such as bradykinin, or even by localized exposure to focused ultrasound. In addition, the permeability of the BBB can be increased by inflammation (either infectious or noninfectious), hypertension, or cerebral ischemia. Moreover, common physiologic processes such as hyperemia (of primary or secondary cause) and neovascularity usually have increased blood volume and blood flow. Abnormal hyperenhancement is usually a result of a combination of increased blood volume/flow and capillary permeability.
Mechanisms of Extra-axial Contrast Enhancement
In extra-axial benign or malignant processes, enhancement occurs because extracerebral vessels (located within the dura mater) do not have a BBB. A leakage of contrast agent is therefore possible in the interstitial compartment and a slightly dural enhancement is seen on MR imaging. After neurosurgery or sometimes lumbar puncture, a diffuse, thick linear dural enhancement may occur and is probably attributable to postintervention inflammatory changes and intracranial hypotension. The pressure exercised on the veins in the subarachnoid space drops when the cerebrospinal fluid pressure is low, enabling vasodilatation and edema. Furthermore, patients having intracranial hypotension are predisposed to subdural hemorrhage after minor trauma as a result of “stretching” of the dural veins.
The most frequent primary extra-axial tumor is the meningioma ( Fig. 2 ): a tumor of meningiothelial cells arising from the arachnoid. This neoplasm is rarely malignant and enhances strongly after contrast medium injection because of the lack of BBB. A thickening of the neighbored dura at the edge of the menigioma is often seen; this is the “dura tail” reflecting edema and congestion of the dura.

Extra-axial malignant tumors show a contrast enhancement because of the lack of BBB and the presence of angiogenesis (see the following section, Mechanisms of Contrast Enhancement in Intra-axial Brain Tumors). The most common extra-axial malignant neoplasm is the metastatic disease carcinomatous meningitis ( Fig. 3 ), usually arising from breast carcinoma in women and prostate cancer in men.

Granulomatous diseases, including sarcoid, tuberculosis, Wegener granulomatous, and rheumatoid nodules, may produce enhancing meningeal nodules without presence of neo-angiogenesis. The mechanisms of enhancement in infectious meningitis are more complex and involve additionally the release of inflammatory mediators facilitating the disruption of the BBB and sometimes leakage of contrast agent in the cerebrospinal fluid.
Mechanisms of Contrast Enhancement in Intra-axial Brain Tumors
In intra-axial primary tumors, mainly gliomas, the BBB can be compromised by neovascularization and direct tumorous damage. Because non-neoplastic astrocytes are required to induce BBB features of cerebral endothelial cells, it is conceivable that malignant astrocytes have lost this ability owing to dedifferentiation. Alternatively, glioma cells might actively degrade previously intact BBB tight junctions. Although the integrity of the barrier is often compromised within the tumor, this alteration in permeability is variable and dependent on the tumor type and size. Moreover, it is extremely heterogeneous in a given lesion ( Fig. 4 ). Although the BBB is frequently leaky in the center of malignant brain tumors, the well-vascularized actively proliferating edge of the tumor, in the brain adjacent to tumor area, has been shown to have variable and complex barrier integrity. In secondary, metastatic intra-axial tumors, the vessels are different from normal cerebral vasculature and have no or strongly disturbed BBB. The enhancement pattern is therefore more homogeneous than in primary tumor.

As brain edema is also thought to be attributable to breakdown of the BBB, one can expect a correlation between the degree of enhancement and the volume of the peritumoral edema. Holodny and colleagues studied this correlation in malignant gliomas as a representative of primary intra-axial tumors and meningeomas as representative of an extra-axial tumor. In their study, no correlation was found for meningeomas, which proved that the meningeoma vessels have no BBB or no effect on the BBB in the surrounding brain tissue. For malignant gliomas, a strong correlation was found, which offers evidence that the defect of the BBB is directly both related to the degree of lesion enhancement and amount of edema and both should interfere between each other. The interference may influence both the conventional contrast-enhanced imaging as well as some of the flow-dependent functional imaging MR techniques. The contrast-enhancement patterns change substantially after corticosteroid treatment, as first presented by the group of Sheffield.
However, the concept of permeability across a disrupted or disturbed BBB in patients with brain tumor has recently gained further interest in monitoring modern treatment strategies. First, changes in permeability may serve as a surrogate marker for other important physiologic processes in brain tumors, such as angiogenesis. Second, an understanding of permeability can elucidate the mechanisms by which therapeutic agents enter brain parenchyma. Third, an understanding of methods for increasing permeability can help in the development of methods to selectively alter the BBB to enhance drug delivery.
Modern MR-neuroimaging strategies, such as dynamic contrast-enhanced MR imaging, focus on the visualization and quantification of the BBB breakdown, which is described in detail in the article on dynamic MR imaging, elsewhere in this issue.
Mechanisms of Contrast Enhancement in Cerebral Infection
Encephalitis
Viral encephalitis usually occurs either as a direct effect of an acute infection, or as one of the sequelae of a latent infection. Sometimes, a serpentine contrast enhancement along gyri can be observed in the subacute phase. In the particular case of herpes virus encephalitis, the contrast enhancement is usually located in the superficial gray matter, often in the medial temporal lobes and in the cingulated gyrus of the medial frontal and parietal lobes. Pathologic specimens reveal spots of petechial hemorrhage and inflammation in almost every case. The slight contrast enhancement is attributable to the inflammatory reaction with altered permeability of the native vessels.
Abscess
Contiguous spread of severe sinus or mastoid infection through the dura is the main cause of pyogenic infections of the brain. Less frequently, cerebral abscesses are attributable to hematogenous septic emboli or occur after trauma. After an initial unorganized, poor demarcated inflammation, the immune system will circumscribe the infection in an abscess, forming a membrane made with granulation tissue and collagen layer, which is surrounded by a layer of astrogliosis. Perifocal edema is always present. The well-defined ring enhancement of the wall is attributable to the increased permeability of the capillaries in the granulation tissue ( Fig. 5 ). In addition, the vascularity is augmented within the abscess wall. Typically, pyogenic abscesses demonstrate restricted diffusion on diffusion-weighted imaging (DWI), owing to the high viscosity of pus, and there is usually a decreased perfusion within the central portion and in the region of surrounding edema. The perfusion is increased only in the contrast-enhanced wall (or in the very closeness of it). Intracranial fungal infection may have findings similar to bacterial infection, and if a patient is immunocompromised, fungal infection should be considered.

Mechanism of Contrast Enhancement in Multiple Sclerosis
The enhancement of multiple sclerosis (MS) plaques is usually seen during the “active phase,” and this enhancement lasts for 2 to 6 weeks and rarely longer. The pathophysiological cause of enhancement in the MS demyelination lesions is attributable to inflammation only. That is why the enhancement of MS plaques is usually faint, because the inflammation is limited to the perivenular/perivascular area, and is not accompanied by angiogenesis or relevant permeability changes of the BBB. This is also the reason why there is usually no perilesional vasogenic edema.
The enhancing rim may be thin and sometimes incomplete with an appearance of an “open ring” sign. This appearance strongly suggests demyelination process and is usually to be distinguished from those of an abscess (which typically has a surrounding edema) and necrotic neoplasm (which has a thick rim). An “incomplete ring” may be seen in active demyelination, both in MS and in tumefactive demyelination. If MS is suspected, MR imaging of the spinal cord may demonstrate additional lesions to help support the diagnosis; however, although imaging may help, the diagnosis of MS remains a clinical diagnosis.
Use of Contrast Media in Stroke Assessment
In the event of suspected acute stroke assessment, a conventional noncontrast brain CT is usually performed before starting any therapy to detect intracerebral hemorrhage that is contraindicated for thrombolytic treatment and also to exclude tumors, vascular malformations, or subdural/extradural hematomas that can imitate the clinical symptoms of stroke. The early signs of acute ischemic stroke can be very subtle, however (hyperdense middle cerebral artery sign, hypoattenuation in basal ganglia, edema in insular cortex, disappearance of gray-white matter discrimination, and midline shift because of edema). Noncontrast CT has limited sensitivity for the diagnosis of ischemic stroke in the initial hours. Improved accuracy of stroke diagnosis, such as perfusion or diffusion-weighted MR imaging is therefore mandatory. Although CT or MRA helps in identification of vascular occlusion and collateral blood supplies, perfusion imaging provides additional information about the extension and hemodynamic status of ischemic tissue.
Use of Contrast Media for Functional Imaging Techniques
Today there are several indications for cerebral perfusion imaging. The major indications in which perfusion MR imaging is routinely used in increasing numbers of imaging centers include cerebrovascular disease, tumor imaging, infectious diseases, epilepsy, and, most recently, psychiatric disorders such as Alzheimer disease and schizophrenia. Because of the radiation dose, perfusion CT is performed mainly in cerebrovascular disease or tumor imaging.
Listing and definition of contrast media indications
Mechanism of Contrast Enhancement in CNS
Owing to the presence of the BBB, the currently available contrast media do not leak into the brain tissue. Only vascular structures and areas of the brain that have no BBB (choroid plexus, pineal and anterior lobe of pituitary gland) physiologically enhance after contrast injection. The BBB consists of a complex of capillary endothelial cells, pericytes, and astroglial and perivascular macrophages and serves as an effective physical barrier to the entry of lipophobic substances into the brain. The BBB blocks all molecules except those that cross cell membranes by means of lipid solubility (such as oxygen, carbon dioxide, ethanol, and steroid hormones) and those that are allowed in by specific transport systems (such as sugars and some amino acids). Substances with a molecular weight higher than 180 Da, which include all available imaging contrast media, generally cannot cross the BBB.
The integrity of the BBB can be altered by a variety of pathologic and physiologic circumstances that increase the permeability, both for contrast media and drug delivery. A disruption of the BBB may be caused by osmotic means (eg, steroids), biochemically by the use of vasoactive substances, such as bradykinin, or even by localized exposure to focused ultrasound. In addition, the permeability of the BBB can be increased by inflammation (either infectious or noninfectious), hypertension, or cerebral ischemia. Moreover, common physiologic processes such as hyperemia (of primary or secondary cause) and neovascularity usually have increased blood volume and blood flow. Abnormal hyperenhancement is usually a result of a combination of increased blood volume/flow and capillary permeability.
Mechanisms of Extra-axial Contrast Enhancement
In extra-axial benign or malignant processes, enhancement occurs because extracerebral vessels (located within the dura mater) do not have a BBB. A leakage of contrast agent is therefore possible in the interstitial compartment and a slightly dural enhancement is seen on MR imaging. After neurosurgery or sometimes lumbar puncture, a diffuse, thick linear dural enhancement may occur and is probably attributable to postintervention inflammatory changes and intracranial hypotension. The pressure exercised on the veins in the subarachnoid space drops when the cerebrospinal fluid pressure is low, enabling vasodilatation and edema. Furthermore, patients having intracranial hypotension are predisposed to subdural hemorrhage after minor trauma as a result of “stretching” of the dural veins.
The most frequent primary extra-axial tumor is the meningioma ( Fig. 2 ): a tumor of meningiothelial cells arising from the arachnoid. This neoplasm is rarely malignant and enhances strongly after contrast medium injection because of the lack of BBB. A thickening of the neighbored dura at the edge of the menigioma is often seen; this is the “dura tail” reflecting edema and congestion of the dura.
Extra-axial malignant tumors show a contrast enhancement because of the lack of BBB and the presence of angiogenesis (see the following section, Mechanisms of Contrast Enhancement in Intra-axial Brain Tumors). The most common extra-axial malignant neoplasm is the metastatic disease carcinomatous meningitis ( Fig. 3 ), usually arising from breast carcinoma in women and prostate cancer in men.
Granulomatous diseases, including sarcoid, tuberculosis, Wegener granulomatous, and rheumatoid nodules, may produce enhancing meningeal nodules without presence of neo-angiogenesis. The mechanisms of enhancement in infectious meningitis are more complex and involve additionally the release of inflammatory mediators facilitating the disruption of the BBB and sometimes leakage of contrast agent in the cerebrospinal fluid.
Mechanisms of Contrast Enhancement in Intra-axial Brain Tumors
In intra-axial primary tumors, mainly gliomas, the BBB can be compromised by neovascularization and direct tumorous damage. Because non-neoplastic astrocytes are required to induce BBB features of cerebral endothelial cells, it is conceivable that malignant astrocytes have lost this ability owing to dedifferentiation. Alternatively, glioma cells might actively degrade previously intact BBB tight junctions. Although the integrity of the barrier is often compromised within the tumor, this alteration in permeability is variable and dependent on the tumor type and size. Moreover, it is extremely heterogeneous in a given lesion ( Fig. 4 ). Although the BBB is frequently leaky in the center of malignant brain tumors, the well-vascularized actively proliferating edge of the tumor, in the brain adjacent to tumor area, has been shown to have variable and complex barrier integrity. In secondary, metastatic intra-axial tumors, the vessels are different from normal cerebral vasculature and have no or strongly disturbed BBB. The enhancement pattern is therefore more homogeneous than in primary tumor.
As brain edema is also thought to be attributable to breakdown of the BBB, one can expect a correlation between the degree of enhancement and the volume of the peritumoral edema. Holodny and colleagues studied this correlation in malignant gliomas as a representative of primary intra-axial tumors and meningeomas as representative of an extra-axial tumor. In their study, no correlation was found for meningeomas, which proved that the meningeoma vessels have no BBB or no effect on the BBB in the surrounding brain tissue. For malignant gliomas, a strong correlation was found, which offers evidence that the defect of the BBB is directly both related to the degree of lesion enhancement and amount of edema and both should interfere between each other. The interference may influence both the conventional contrast-enhanced imaging as well as some of the flow-dependent functional imaging MR techniques. The contrast-enhancement patterns change substantially after corticosteroid treatment, as first presented by the group of Sheffield.
However, the concept of permeability across a disrupted or disturbed BBB in patients with brain tumor has recently gained further interest in monitoring modern treatment strategies. First, changes in permeability may serve as a surrogate marker for other important physiologic processes in brain tumors, such as angiogenesis. Second, an understanding of permeability can elucidate the mechanisms by which therapeutic agents enter brain parenchyma. Third, an understanding of methods for increasing permeability can help in the development of methods to selectively alter the BBB to enhance drug delivery.
Modern MR-neuroimaging strategies, such as dynamic contrast-enhanced MR imaging, focus on the visualization and quantification of the BBB breakdown, which is described in detail in the article on dynamic MR imaging, elsewhere in this issue.
Mechanisms of Contrast Enhancement in Cerebral Infection
Encephalitis
Viral encephalitis usually occurs either as a direct effect of an acute infection, or as one of the sequelae of a latent infection. Sometimes, a serpentine contrast enhancement along gyri can be observed in the subacute phase. In the particular case of herpes virus encephalitis, the contrast enhancement is usually located in the superficial gray matter, often in the medial temporal lobes and in the cingulated gyrus of the medial frontal and parietal lobes. Pathologic specimens reveal spots of petechial hemorrhage and inflammation in almost every case. The slight contrast enhancement is attributable to the inflammatory reaction with altered permeability of the native vessels.
Abscess
Contiguous spread of severe sinus or mastoid infection through the dura is the main cause of pyogenic infections of the brain. Less frequently, cerebral abscesses are attributable to hematogenous septic emboli or occur after trauma. After an initial unorganized, poor demarcated inflammation, the immune system will circumscribe the infection in an abscess, forming a membrane made with granulation tissue and collagen layer, which is surrounded by a layer of astrogliosis. Perifocal edema is always present. The well-defined ring enhancement of the wall is attributable to the increased permeability of the capillaries in the granulation tissue ( Fig. 5 ). In addition, the vascularity is augmented within the abscess wall. Typically, pyogenic abscesses demonstrate restricted diffusion on diffusion-weighted imaging (DWI), owing to the high viscosity of pus, and there is usually a decreased perfusion within the central portion and in the region of surrounding edema. The perfusion is increased only in the contrast-enhanced wall (or in the very closeness of it). Intracranial fungal infection may have findings similar to bacterial infection, and if a patient is immunocompromised, fungal infection should be considered.