Uterine leiomyomas, also known as myomas or fibroids , are common neoplasms of the uterine myometrium. They are benign monoclonal tumors of smooth muscle, with variable amounts of connective tissue. Leiomyomas may be asymptomatic or cause symptoms such as bleeding, pain, and urinary difficulties. We will focus on the imaging aspects of uterine leiomyomas. Discussion of the clinical aspects and treatment options is beyond the scope of this chapter, although we will discuss a few aspects of treatment that are related to imaging. Other chapters in the book are more specifically devoted to imaging-dependent treatment methods.
There is a clinical syndrome of which one should be aware, however, because, despite its rarity, there is a higher level of clinical concern in affected patients. Patients with hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome have mutations in the fumarate hydratase gene, and have variable presence of uterine leiomyomas, cutaneous leiomyomas, and renal cell carcinoma. The uterine leiomyomas in patients with this syndrome tend to produce more symptoms than those in the general population. Renal cell carcinoma occurs in a little less than 25% of patients with the syndrome but, when present, is often aggressive. Women with the syndrome have an increased risk for uterine leiomyosarcoma (LMS). The HLRCC syndrome should be suspected in a patient with cutaneous leiomyomas and a striking personal or family history of highly symptomatic uterine leiomyomas.
The growth rate of uterine leiomyomas in premenopausal women varies widely, with a median growth rate of 9% at 6 months. Growth rates of leiomyomas vary within the same patient, are not influenced by size or location of the leiomyoma, and spontaneous regression of leiomyomas may occur. The growth rate of leiomyomas is similar in black and white women up to 35 years of age but slower in white women thereafter. During pregnancy the growth rate of uterine leiomyomas is also variable, but it appears that most leiomyomas do not enlarge during pregnancy. Of the minority that do enlarge, most of the growth occurs before 10 weeks of gestational age. Leiomyomas that enlarge during pregnancy usually shrink after the pregnancy.
Location of Leiomyomas
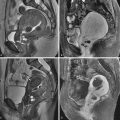
Uterine leiomyomas are often separated into three general groups based on location: submucosal, intramural, and subserosal ( Figure 15-1 ). The distinction among them is not always clear because there is sometimes overlap and large leiomyomas may span all three locations. The term submucosal is commonly used, although it has been suggested as a misleading term because the uterus has no submucosal layer. Submucosal leiomyomas are often subclassified by the schema of the European Society of Hysteroscopy and typically include any leiomyomas that touch the endometrium. A type 0 leiomyoma is completely within the endometrial cavity; type I has more than 50% of the leiomyoma within the endometrial cavity; and type II has less than 50% of the leiomyoma within the endometrial cavity. Type 0 and type I submucosal leiomyomas can usually be safely resected by hysteroscopy. Type II submucosal leiomyomas may sometimes be removed hysteroscopically, depending on the skill and experience of the hysteroscopist and the amount of surrounding myometrium. Intramural leiomyomas are those completely surrounded by myometrium. Subserosal leiomyomas abut the uterine serosa and have partial or complete absence of surrounding myometrium. It has been suggested that pedunculated subserosal leiomyomas be defined as those where the center of the leiomyoma is outside the uterus, and in which the stalk diameter is less than 50% of the diameter of the leiomyoma. This definition, although not unreasonable, is somewhat arbitrary and problematic. It does not address whether the maximum, mean, or some other diameter of either the stalk or leiomyoma is considered. Additionally, data are lacking on the accuracy and reproducibility of stalk diameter determination by imaging methods.

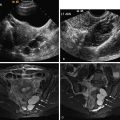
Pedunculated subserosal leiomyomas are not uncommon. When presented with a solid adnexal mass that does not clearly arise from the ipsilateral ovary, a pedunculated leiomyoma should be a strong consideration. The stalk may be difficult to identify by gray scale ultrasound (US). Color or power Doppler US should be used to search for the connecting vessels in the stalk ( Figure 15-2 ). Leiomyomas may occasionally occur in other locations such as the cervix or broad ligament.

Typical Imaging Findings of Leiomyomas
Leiomyomas are best imaged by US or magnetic resonance imaging (MRI). It is fortunate that these two modalities do not use ionizing radiation. Leiomyomas may be seen with other imaging modalities, but these other modalities are generally not optimal. We will review appearances of typical leiomyomas with the different imaging modalities. Less common appearances such as those that occur with degeneration and histologic variants will be discussed subsequently.
Ultrasound
US is usually the first method of imaging when one wants to identify leiomyomas. US is generally reliable for identifying or excluding leiomyomas. With large or multiple leiomyomas, US may have difficulty identifying all the leiomyomas, and reliable measurements may be difficult. Tiny leiomyomas may also escape detection by US.
Both transabdominal and transvaginal US are often needed to adequately evaluate the uterus. Transvaginal scanning performs well for small- to moderate-sized leiomyomas. Large or pedunculated leiomyomas may be missed by transvaginal scanning alone. Other sonographic techniques such as sonohysterography and three-dimensional US may aid in better showing the submucosal extent of leiomyomas if the location is not clear on conventional two-dimensional US ( Figure 15-3 ).

Leiomyomas typically appear as solid masses that are hypoechoic compared with the normal myometrium ( Figure 15-4 ). They are often slightly heterogeneous and usually have variable degrees of acoustic shadowing and refractive artifacts. Occasionally leiomyomas may be isoechoic or hyperechoic compared with normal myometrium. Calcification, appearing as brightly echogenic foci with distal acoustic shadowing, occurs in a minority of leiomyomas. Color or power Doppler US is reported to show mostly peripheral flow, but leiomyomas may occasionally have central flow present (see Figure 15-4 ).

Measurement of leiomyoma size is usually straightforward with US, but there are potential pitfalls, particularly when following leiomyomas for size change. If there are only a few small leiomyomas, there usually is little problem. When the leiomyomas are large and/or numerous, it may be difficult to obtain accurate measurements. Accurate serial measurement by US may be hampered by subjectivity in determining borders of a leiomyoma, selection of different planes for measurement, and occasional difficulty in determining whether the lesion is one leiomyoma versus multiple adjacent leiomyomas. One may be tempted to always use measurements obtained with transvaginal scanning, thinking they may be more accurate. Leiomyomas, particularly when large, however, are sometimes better seen and measured with transabdominal scanning. One will often need to look carefully at the images of the measurements to ensure that the same leiomyoma is compared and that the measurement planes and technique are consistent.
Magnetic Resonance Imaging
Compared with US, MRI is more accurate for size, number, and location of leiomyomas, particularly when they are numerous and/or large. Compared with normal myometrium, most leiomyomas are isointense on T1-weighted imaging and hypointense on T2-weighted imaging ( Figure 15-5 ). A thin high signal rim (see Figure 15-5 ) is sometimes present on T2-weighted imaging and may be caused by lymphatics, edema, or dilated veins. After intravenous administration of gadolinium-based contrast agents, leiomyomas usually enhance the same or slightly less than myometrium (see Figure 15-5 ). Occasionally leiomyomas may enhance more than myometrium. With large leiomyomas, compression by the sacral promontory may cause diminished enhancement in the adjacent area.

Computed Tomography
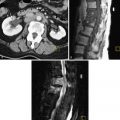
Computed tomography (CT), aside from the issue of ionizing radiation, is not a primary imaging method for leiomyomas because of the lack of sufficient contrast resolution in most cases. Given the common use of CT, however, leiomyomas may be identified. One may observe an enlarged uterus, altered uterine contour, or the mass of a leiomyoma ( Figure 15-6 ). The attenuation of leiomyomas is similar to myometrium on CT scans without an intravenous contrast agent. After administration of an intravenous contrast agent, most leiomyomas enhance similar to, or slightly less than, the myometrium. As with MRI, occasional leiomyomas may enhance more than the myometrium.

Positron Emission Tomography
There is no extensive experience yet with positron emission tomography (PET) of uterine leiomyomas. Preliminary evidence suggests that a minority of leiomyomas can have uptake on fluorine-18 fluorodeoxyglucose (FDG)/PET scanning ( Figure 15-7 ). In one study with leiomyoma determination based on US or MRI, 14% of leiomyomas had uptake greater than the liver. In another study, also lacking pathologic correlation, 10% of premenopausal women and 1% of postmenopausal women had leiomyomas with FDG uptake. Leiomyomas with uptake often correlated with high signal intensity on T2-weighted MRI. Interestingly, on serial studies, FDG activity could change and/or there could be new activity on a later study when it was not present initially. This latter study also suggested that uptake may be more common in the luteal phase of the menstrual cycle. These studies were limited, however, because no histologic confirmation of leiomyomas was available. We do not yet know whether certain types of leiomyomas, such as cellular leiomyomas, have FDG uptake more commonly than typical leiomyomas.

Other Radiographic Imaging Methods
Conventional radiographic imaging is rarely helpful for imaging leiomyomas. Occasionally calcified leiomyomas or soft tissue mass effect from a large leiomyoma may be evident on conventional radiographs of the pelvis. Hysterosalpingography is not a primary method to image leiomyomas, although secondary mass effect on the endometrium may be evident. Angiography is not typically used to image leiomyomas, other than as part of uterine artery embolization (UAE).
Alternative Diagnoses to Consider
The vast majority of myometrial masses are leiomyomas, and their diagnosis is usually straightforward using US or MRI. Occasionally alternative diagnoses should be considered. Adenomyosis seems to be the most common lesion that is mistaken for a leiomyoma. Adenomyosis usually has less distinct margins than a leiomyoma and is generally a diffuse process, although it can be focal. A discussion of the imaging features of adenomyosis is available in Chapter 14 . Myometrial contractions may also simulate a leiomyoma. Contractions are most common during pregnancy but have also been described in nonpregnant patients. Contractions can be recognized by their temporal change, as they usually change shape over the course of several minutes and almost always resolve within approximately 30 minutes, if not sooner. LMS is another consideration for a myometrial mass and will be discussed in more detail subsequently.
Centrally located leiomyomas might be confused for endometrial pathology. Compared with myometrium, most leiomyomas are hypoechoic on US and have moderately low signal on T2-weighted MRI sequences, as opposed to endometrial polyps, which are typically hyperechoic on US and have only slightly low signal on T2-weighted MRI sequences. Hyperechoic leiomyomas occur occasionally and are usually caused by lipoleiomyomas, which will be discussed subsequently. If US demonstrates a hyperechoic mass centrally in the uterus and the endometrium is not clearly seen, a hyperechoic leiomyoma could be confused with an endometrial polyp or with retained products of conception in the appropriate clinical setting. Sonohysterography may be helpful to determine whether the mass is endometrial or myometrial in origin.
When faced with a suspected pedunculated subserosal leiomyoma, particularly when the ipsilateral ovary is not seen, color or power Doppler US demonstration of a vascular pedicle (see Figure 15-1 ) connecting it to the uterus is generally sufficient for diagnosis, as discussed previously. If one does not see the pedicle, then alternative diagnoses should be considered. MRI is usually helpful in these cases. Ovarian neoplasms, particularly those from the sex cord/stromal group, are often solid, and a fibroma is the most common such neoplasm. Tumors arising from other organs such as the bowel or nerves should also be considered. The rudimentary horn of a unicornuate uterus may also appear as a solid mass.
Calcifications, likely in arcuate vessels, may be seen in the outer myometrium of older patients. With US, these typically appear as multiple small echogenic foci in the periphery of the uterus and should not be confused with calcified leiomyomas. When leiomyomas calcify, they typically appear as larger clumps of calcification or as a shell of calcification around the periphery of the leiomyoma.
Degenerated Leiomyomas
Degeneration may not necessarily be the most appropriate term but has been used to describe various types of benign histologic change in leiomyomas. The different forms of degeneration occur with variable frequency and usually do not carry much clinical significance. Their importance generally lies in recognizing the imaging features and not mistaking them for something more sinister. Identification of the various forms of degeneration based on imaging findings, however, can be problematic. Some types of degeneration are more readily identified on MRI than on US. The studies that have described the imaging findings of degeneration (and leiomyoma variants, as discussed subsequently) have found somewhat variable results, and variability in the histologic diagnosis may also hinder our understanding of the appearance of degenerated and variant leiomyomas. Thus it may be difficult to precisely determine the type of degeneration in all cases. The appearances discussed below are based on what seems to be the preponderance of features described in the literature.
Hyaline Degeneration
Hyaline degeneration occurs when smooth muscle is replaced by fibrous connective tissue and is the most common form of degeneration in leiomyomas. Although often considered to be the result of hypoxia from outgrowth of blood supply, this concept may not be correct because hyaline degeneration also occurs in small leiomyomas. Hypoestrogenism or senescence may play a role. Variable imaging features have been reported. There often is little to no recognizable change on US. With MRI, leiomyomas with hyaline degeneration tend to have lower signal intensity on T2-weighted imaging ( Figure 15-8 ) and less enhancement after administration of gadolinium agents compared with usual leiomyoma. More extensive hyalinization tends to produce poorer enhancement. Low signal on T2-weighted imaging with a speckled appearance has occasionally been described with hyaline degeneration. The difference in lower signal intensity on T2-weighted imaging, at least for leiomyomas with milder degrees of hyalinization, is often small compared with usual leiomyomas, and it is not clear how reliably one can make this determination with imaging studies.