Fig. 19.1
Example of mediastinal and cardiac region of interest (ROI) on anterior planar image. The left ventricular ROI was drawn including the ventricular cavity (H), and a rectangular ROI was set on the middle mediastinum (M)
Though several medications can interfere with MIBG uptake, among anti-Parkinson drugs particularly L-threo-DOPS and MAO-B inhibitors may reduce the cardiac MIBG uptake (Solanki et al. 1992) (Table 19.1). For reliable results, the use of these drugs should be avoided.
Table 19.1
Drugs interacting with MIBG
Antidepressants |
Amitriptyline, amoxapine, clomipramine, desipramine, imipramine, trazodone, mianserin, etc. |
α- and β-blocker |
Labetalol, phenoxybenzamine, etc. |
Sympathomimetics and sympatholytics |
L-threo-DOPS, norepinephrine, dobutamine, dopamine, phenylephrine, phenylpropanolamine, salbutamol, selegiline, brimonidine, etc. |
Antipsychotics |
Chlorpromazine, trazodone, haloperidol, droperidol, clozapine, quetiapine, risperidone, etc. |
CNS stimulants |
Cocaine, caffeine, amphetamine, etc. |
Calcium channel blocker |
Diltiazem, isradipine, nicardipine, nifedipine, nimodipine, verapamil, etc. |
It is well known that various type of cardiac diseases and diabetes mellitus exhibited low MIBG uptake. In those cases, distribution pattern of MIBG accumulation may help the differential diagnosis. Three-dimensional evaluation using SPECT and ecocardiographic assessment for cardiac wall motion is recommended to confirm the cause of MIBG uptake reduction (Nakajima et al. 2008).
19.3 Pathology in Lewy Body Disease
Pathological assessment of Lewy body disease (LBD) reveals aggregates of α-synuclein, referred to as Lewy bodies, found in neurons and glia. Their presence is required for the pathological diagnosis of LBD. Lewy bodies are present not only in the brain stem but also in the limbic system and neocortex in relation to their phenotypes. In addition to central nervous system involvement, variable amounts of Lewy body burden are found in the peripheral nervous system and autonomic nervous system (Wakabayashi and Takahashi 1997). A postmortem study reported cardiac plexus was more heavily involved than the sympathetic ganglia in patient with PD (Mitsui et al. 2006). On the other hand, other movement disorder and dementia syndromes, such as progressive supranuclear palsy (PSP), multiple system atrophy (MSA), essential tremor, frontotemporal dementia, and Alzheimer’s-type dementia (AD), generally found no sympathetic ganglionic changes. Accordingly, Lewy body disease is not specific to the brain but instead is a generalized neuronal disorder.
19.4 Meta-iodobenzylguanidine Imaging in Lewy Body Disease
PD, DLB and pure autonomic failure (PAF) are recognized as LBD. Among all neurological diseases, they are characterized by a significant reduction in MIBG uptake. Recently, a markedly reduced cardiac MIBG uptake was also reported in idiopathic rapid eye movement sleep behavior disorder (RBD), consistent with the loss of sympathetic terminals and suggesting an association of RBD with Lewy body pathology (Miyamoto et al. 2006) (Fig. 19.2). As 123I-N-ω-fluoropropyl-2β- carbomethoxy-3β-(4-iodophenyl)nortropan (FP-CIT) SPECT successfully visualizes presynaptic dopaminergic degeneration of the nigrostriatal tract, the finding of reduced tracer uptake in basal ganglia is recognized as a feature “suggestive” of DLB (Mckeith et al. 2005) (Table 19.2). A study aimed at differentiation of PD from atypical parkinsonian disorder revealed that 123I-FP-CIT SPECT and MIBG scintigraphy have similar diagnostic accuracy (Südmeyer et al. 2011).

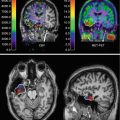
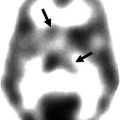
Fig. 19.2
Brain 18F-FDG -PET and anterior planar MIBG image of patients with REM sleep behavior disorder. 18F-FDG-PET shows normal brain metabolism, but no cardiac MIBG uptake (arrows)
Table 19.2
FP-CIT SPECT and MIBG scintigraphy in discrimination of DLB from AD
Tracer | Diagnostic significance (McKeit et al. 2005) | Subject | Method | Sensitivity/specificity (%) |
|---|---|---|---|---|
FP-CIT | Suggestive feature | Probable DLB vs. non DLB | Multicenter | 77.7∕90.4 (McKeitet al. 2007) |
MIBG | Supportive feature | Probable DLB vs. probable AD | Single center |
19.4.1 Parkinson’s Disease
MIBG studies for PD began in 1994 (Hakusui et al. 1994; Yoshita et al. 1996; Iwasa et al. 1998). Their clinical value has become recognized by an increasing number of reports and experiences (Yoshita 1998; Braune et al. 1998; Orimo et al. 1999; Reinhardt et al. 2000; Druschky et al. 2000; Takatsu et al. 2000a, b; Taki et al. 2000). A characteristic feature of MIBG imaging in PD has been shown to be an impressively low uptake in the whole myocardium. The severely depressed cardiac MIBG uptake in PD seems to occur in a disease-specific manner among related movement disorders. Based on a meta-analysis of studies, the overall sensitivity and specificity for correct discrimination of patients with PD were both about 90 % (Braune 2001; Orimo et al. 2012; Treglia et al. 2012).
Although the H/M ratio tends to decrease with disease progression, many PD patients in Hoehn and Yahr stage I have decreased cardiac MIBG uptake. The H/M ratio is not suitable as an index of the disease severity. Tremor-dominant patients with PD had higher MIBG uptake than those with postural instability and gait disorder, while reduced MIBG uptake was linked to greater bradykinesia, higher age at onset, and longer disease duration (Saiki et al. 2004; Spiegel et al. 2007).
Cardiac MIBG uptake is not correlated with dysautonomic clinical symptoms in patients with PD, though some cardiac autonomic tests showed a negative correlation of MIBG uptake and cardiac sympathetic denervation (Oka et al. 2011). The use of different methods for evaluation of cardiac MIBG uptake or early occurrence of sympathetic nerve fiber loss in LBD might explain such poor correlations (Iwanaga et al. 1999; Taki et al. 2004).
In genetically identified cases of PD, cardiac MIBG uptake findings are not consistent. MIBG or fluorodopamine uptake in the heart decreased in some patients with PARK1, PARK4, PARK6, PARK7, and PARK 8 mutations (Quattrone et al. 2008; Valldeoriola et al. 2011; Tijero et al. 2010). On the contrary, patients with PARK2 mutations showed no difference in H/M ratios as compared to controls (Orimo et al. 2005).
19.4.2 Dementia with Lewy Bodies
Antemortem diagnosis of DLB and differentiation of DLB from AD is important, because some patients with DLB may show an accelerated disease progression, life-threatening adverse reaction to antipsychotic medications, and a good response to anticholinesterase inhibitors. Clinical utility of MIBG scintigraphy in differentiating DLB from AD has been reported (Watanabe et al. 2001; Yoshita et al. 2001, 2006; Treglia and Gason 2012). Although the diagnosis of DLB may be confounded by the absence of parkinsonism, DLB patients without parkinsonism had a decreased MIBG accumulation in the heart as well as those with parkinsonism (Yoshita et al. 2006) (Fig. 19.3). The MIBG uptake reduction does not correlate with disease duration. Consensus guidelines for the clinical and pathologic diagnosis of DLB were published in 2005. In these guidelines, in addition to the central, core, and suggestive features, abnormally low MIBG uptake in myocardial scintigraphy was suggested as one of the supportive features (McKeith et al. 2005).
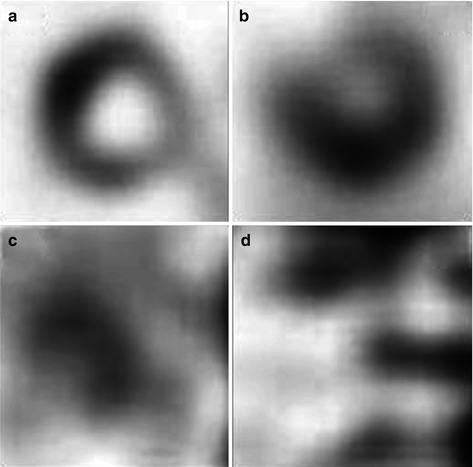

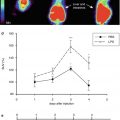
Fig. 19.3
Cardiac radioactivity in delayed short axis view after injection of 123I-MIBG in a control subject, a patient with AD, a patient with DLB with parkinsonism, and a patient with DLB without parkinsonism. Both patients with DLB have undetectable myocardial 123I-MIBG radioactivity. (a) Control, (b) AD, (c) DLB with parkinsonism, (d) DLB without parkinsonism
Occipital hypoperfusion on brain perfusion SPECT is a “supportive” feature of DLB as well as depressed MIBG cardiac accumulation (McKeith et al. 2005). However, the difference in cardiac uptake of MIBG between DLB and AD seemed to be more intuitively recognizable (Table 19.3). When the MIBG study was compared with brain perfusion SPECT with 99mTc-ethylene cysteine dimer or 123I-N-isopropyl-iodoamphetamine, the MIBG study seemed to be more accurate as a means of discriminating DLB from AD (Yoshita and Yamada 2003; Hanyu et al. 2006). Although brain perfusion SPECT failed to demonstrate significant hypoperfusion in the occipital region in some patients with DLB, a marked reduction of MIBG uptake was found in most of the patients with DLB.
Table 19.3
Discriminating DLB from AD using brain perfusion SPECT
Tracer | Subjects | Sensitivity/specificity (%) |
|---|---|---|
99mTc-HMPAO | 23 DLB (definite 4, probable 17, possible 2) vs. 50AD (definite 2, probable 21, possible 27) | 65∕87 (Lobotesis et al. 2001) |
123I-IMP | 19 probable DLB vs. 39 probable AD | 74∕82 (Hanyu et al. 2006) |
99mTc-ECD | 23 probable DLB vs. 23 probable AD | 65∕75 (Yoshita and Yamada 2003) |
19.4.3 Pure Autonomic Failure
PAF is a rare phenotype of Lewy body disease in which patients have prominent autonomic disturbance thought to be associated with a diffuse loss of sympathetic terminal innervations. PET with 6-18F-flurodopamine as well as MIBG studies revealed a loss of cardiac tracer uptake, indicating cardiac denervation in PAF (Goldstein et al. 2000; Yoshida et al. 1997). 123I-MIBG cardiac scintigraphy should be able to distinguish MSA and PAF (Hirayama et al. 1995; Kashihara et al. 2006).
19.5 Differential Diagnosis of Lewy Body Disease Using 123I-MIBG Cardiac Scintigraphy
In general, a neurological differential diagnosis in the early stages between PD, MSA, PSP, and corticobasal degeneration (CBD) is important because of differences in prognosis and therapy among these diseases. In contrast with PD, central and preganglionic neurons are predominantly affected, while postganglionic neurons are usually spared in MSA. Therefore, cardiac MIBG uptake might not be impaired. In MSA, cardiac MIBG uptake is higher than that in PD, irrespective of the severity of autonomic dysfunction (Yoshita 1998; Braune et al. 1999; Orimo et al. 1999). Although 77–95 % discrimination specificity between PD and MSA has been proposed (Braune 2001; Treglia et al. 2011), the Quality Standards Subcommittee of the American Academy of Neurology found insufficient evidence to recommend MIBG cardiac imaging for differentiating PD from MSA (Suchowersky et al. 2006). A few cases of patient with MSA showed decreased cardiac MIBG uptake (Yoshita 1998, 2000). A small number of patients with MSA have postmortem evidence of postganglionic cardiac denervation which may account for the reported decreased cardiac MIBG uptake (Orimo et al. 2007). These cases of MSA may have involvement of both glial cytoplasmic inclusion in the brain and Lewy bodies in sympathetic ganglia.
PSP, classified as tauopathy, is a degenerative disorder causing supranuclear gaze palsy, bradykinesia, muscular rigidity with progressive axial dystonia, pseudobulbar palsy, and subcortical dementia. The H/M ratio of this disease entity is within normal range or slightly reduced (Yoshita 1998; Taki et al. 2000). However, we have noted a markedly low MIBG uptake in about 20 % of patients with PSP (Yoshita et al. 2008) (Table 19.4, Fig. 19.4). These cases of PSP may have concomitant Lewy bodies (Mori et al. 2002).
Table 19.4
Demographic and clinical data
PSP | CBD | PD | DLB | CN | p value | |
|---|---|---|---|---|---|---|
N (M/F) | 19 (13∕6) | 7 (2∕5) | 19 (8∕11) | 11 (5∕6) | 13 (6∕7) | NA |
Duration (mo) | 29.4 ± 20.9 | 18.4 ± 12.5 | 67.4 ± 61.6 | 36.3 ± 24.6 | NA | <0.01a |
Age (y)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|