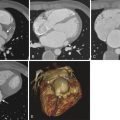
Echocardiography is the primary imaging method for the evaluation of patients suspected of having valvular dysfunction because this modality is relatively inexpensive, fast, and available in most hospitals. Its temporal resolution allows accurate depiction of valve morphology. However, echocardiography has limitations, including nondiagnostic or limited scans secondary to patient body habitus or emphysema, as well as interobserver variability of measurements of ejection fraction (EF) and valvular regurgitation.
Magnetic resonance imaging (MRI) is helpful to characterize valvular dysfunction further in certain patients. It is more accurate for quantifying ventricular mass and function, information that aids the cardiologist and surgeon in determining the appropriate timing of valvular surgery. Velocity encoded cine (VEC) phase contrast MRI allows highly accurate and reproducible quantification of regurgitant fraction in the setting of valvular insufficiency and pressure gradients across a valvular stenosis. In this chapter, the role of MRI in various conditions and diseases affecting the cardiac valves is explored ( Box 35-1 and Table 35-1 ).
Assessment of severity of stenosis or regurgitation
Measurement of effects of volume overload on the heart and degree of myocardial hypertrophy
Assessment for complications of valvular endocarditis
Diagnosis and definition of valvular mass seen on echocardiography or computed tomography
Evaluation of postsurgical tetralogy of Fallot
Diagnosis of systolic anterior motion of the mitral valve and left ventricular outflow tract obstruction in hypertrophic cardiomyopathy
| Imaging View | Information Obtained |
|---|---|
| Longitudinal and perpendicular images through valve | Valve morphology and opening pattern |
| Outflow tract | Identification of stenosis or regurgitation |
| Cine four-chamber | Global cardiac wall motion |
| Phase contrast imaging perpendicular to valvular plane and proximal or distal to valve | Measurement of valvular gradient and construction of flow volume curves |
| Short axis | End-diastolic volume, end-systolic volume, and ejection fraction |
| Planimetry perpendicular to valve | Valve area |
| Late or delayed enhancement | Myocardial viability |
Clinical Considerations
Anatomy and Physiology
The four cardiac valves comprise two atrioventricular (mitral and tricuspid) and two semilunar (aortic and pulmonic) valves ( Figs. 35-1 to 35-4 ). The aortic and mitral valves are adjacent and in fibrous continuity, whereas the pulmonic and tricuspid valves are separated by the muscular infundibulum of the right ventricle ( Figs. 35-5 and 35-6 ).






Normal cardiac valves allow unidirectional unimpeded blood flow. If regurgitation or stenosis develops, the ventricle compensates with either dilatation or muscular hypertrophy. A cardinal rule of cardiology states that valvular regurgitation results in a dilated ventricle because of volume overload, whereas valvular stenosis results in a hypertrophied ventricle because of pressure overload. At first, these mechanisms compensate for the valvular abnormality, but over time, the ventricle eventually fails.
Clinical Pearl
Valvular regurgitation results in ventricular dilatation, and valvular stenosis results in ventricular hypertrophy.
Systematic Approach to Valvular Abnormalities
Several questions are important to address in the imaging evaluation of the valves:
- 1.
How does the valve look?
Valve anatomy (unicuspid, bicuspid, tricuspid leaflets) ( Fig. 35-7 )

Figure 35-7
Axial turbo field echo image shows a bicuspid aortic valve (arrow).
Leaflet morphology (prolapsed, ruptured, fused, calcification, thickening, vegetation)
Perivalvular abnormality (aneurysm, abscess)
Valve cross-sectional area
- 2.
How does the valve function?
Regurgitation or stenosis
Opening and closing pattern
- 3.
How does the valve affect cardiac function?
EF and diastolic filling
Wall motion
Cardiac volumes
Cardiac wall mass
- 4.
Should additional points be addressed in the assessment of this particular valvular problem?
Cardiac thrombus
Coronary arterial disease
Myocardial viability
Associated conditions such as coarctation of the aorta with bicuspid aortic valve ( Fig. 35-8 )

Figure 35-8
Coarctation of the aorta.
Contrast-enhanced oblique sagittal magnetic resonance angiogram image through the aorta reveals narrowing (arrow) just distal to the takeoff of the left subclavian artery typical of coarctation. Velocity encoded cine phase contrast techniques can be used to measure the pressure gradient across the stenosis and collateral flow around the narrowing. Coarctation and pseudocoarctation are associated with a bicuspid aortic valve.
Regurgitation
Valvular regurgitation may have acute or chronic causes. Acute regurgitation is poorly tolerated, causes sudden and often severe symptoms, and is diagnosed primarily with echocardiography. It is rarely imaged with MRI. An example of a situation in which acute regurgitation is encountered is in the setting of mitral valve prolapse resulting from postinfarct papillary muscle rupture. This discussion focuses on chronic regurgitation.
Chronic mitral regurgitation is the most common valvular abnormality in industrialized countries. Its causes are myriad and include mitral valve prolapse, cardiomyopathies (ischemic, nonischemic, and hypertrophic obstructive [HCM]), rheumatic heart disease, and, rarely, left atrial myxoma. Mitral valve prolapse is the most common congenital cause of mitral regurgitation, characterized by myxomatous degeneration of the valvular apparatus without identifiable systemic connective tissue disease. It is defined by demonstration of leaflet bowing of greater than 2 mm beyond the valvular plane and by a maximal leaflet thickness greater than 5 mm ( Figs. 35-9 to 35-11 ). Most patients with mitral regurgitation are asymptomatic until the development of atrial fibrillation, pulmonary hypertension, or ventricular failure.



Chronic aortic regurgitation is most often secondary to idiopathic degeneration and frequently occurs in conjunction with aortic stenosis. In patients less than 40 years old, Marfan syndrome with annuloaortic ectasia is the most frequent cause. Causes of aortic regurgitation are listed in Table 35-2 . Symptoms secondary to aortic regurgitation include dyspnea, angina, and palpitations. Diagnosing aortic regurgitation before symptom onset is important because symptoms often herald the presence of irreversible left ventricular damage, a situation in which valvular replacement is no longer an effective treatment.
| Leaflet Problems | Aortic or Annular Dilatation |
|---|---|
| Bicuspid aortic valve | Annuloaortic ectasia |
| Rheumatic valvular disease | Marfan syndrome |
| Myxomatous degeneration | Hypertension |
| Rheumatoid arthritis | Aortic aneurysm |
Pulmonary regurgitation and tricuspid regurgitation are most commonly associated with pulmonary arterial hypertension or congenital heart disease. Pulmonary regurgitation associated with repaired tetralogy of Fallot is a special situation and is described later. Patients are usually asymptomatic from these conditions unless regurgitation is severe. Severe pulmonic or tricuspid regurgitation manifests with symptoms of right ventricular failure, including ascites, fatigue, and lower extremity swelling.
Pathophysiology
Valvular regurgitation leads to a volume overload state in the heart. The body compensates with chamber dilatation that preserves the effective stroke volume and cardiac output initially. Over time, this adaptive response leads to irreversible ventricular damage or atrial dilation, resulting in symptoms.
Magnetic Resonance Imaging
Most patients have a diagnosis of valvular regurgitation before they present for cardiac MRI. Occasionally, regurgitation is identified during the imaging evaluation for other indications, and the examiner must recognize the MRI appearance of a regurgitant valve. Currently, cine steady-state free precession (SSFP) is the primary technique used for the evaluation of cardiac valves. This sequence offers an excellent signal-to-noise ratio and high contrast between the blood pool and the myocardium. A disadvantage compared with cine fast gradient echo imaging is that a shorter echo time (TE) is used with SSFP. This disadvantage can be important because detection of a regurgitant flow jet depends on spin dephasing artifact secondary to turbulent flow. As the TE for the sequence decreases, turbulent flow from regurgitation has less dephasing artifact and creates less signal void. This issue is not necessarily significant in most patients undergoing valvular evaluation by MRI because they already carry a diagnosis of regurgitation.
Regurgitation shows a signal void directed away from the closed valve back into the chamber during diastole ( Figs. 35-12 to 35-15 ). The size of the regurgitant jet is not an accurate quantifier of regurgitation because it varies with the TE value used in image acquisition and reflects turbulent blood flow and not the volume of regurgitation.




Clinical Pitfall
A TE value of less than 6 msec may not accurately depict a stenotic or regurgitant jet.
Quantification of Regurgitation in Patients With One Dysfunctional Valve
After identifying a regurgitant valve, the next step is quantification of the volume of regurgitation. This is accomplished with the use of either VEC MRI (the phase contrast technique) for direct quantification of retrograde diastolic flow or cine MRI to compare the stroke volumes of the two ventricles. In patients without regurgitation, the stroke volume of the right ventricle is equal to the stroke volume of the left ventricle. In patients with a single regurgitant valve, the difference in ventricular stroke volume is equal to the regurgitant volume. In any given patient, measurements using the two techniques can be compared, to serve as an internal control.
Quantification of Regurgitation in Patients With More Than One Dysfunctional Valve and Construction of a Flow Volume Curve
VEC MRI is the most widely used method to quantify regurgitation in patients with more than one regurgitant valve. The technique is similar for aortic and pulmonic regurgitation.
Velocity Encoded Cine Phase Contrast Technique
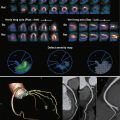
Information from the phase contrast sequence yields both magnitude and phase images. The velocity of flowing blood is encoded in each voxel, depicted in grayscale on the phase images ( Fig. 35-16 ). The magnitude images are a bright-blood gradient echo sequence used to define the anatomy and locate the vessel or outflow tract of interest. The vessel wall is a reference point for zero velocity, whereas flowing blood is depicted with varying degrees of white or black shading, depending on the velocity and direction of flow.

A region of interest (ROI) is drawn around the aorta or pulmonary artery perpendicular to flow and just superior to the valvular plane on each anatomic magnitude image. This ROI is then applied to the related phase image. Each voxel within the ROI has a specific encoded velocity (Venc) that is depicted by the corresponding grayscale value. The average velocity of the vessel of interest, measured just above the valvular plane, can be determined by measuring the cross-sectional area of the vessel; the product of vessel area and average mean velocity yields the flow volume for that particular frame of the cardiac cycle:
Vessel area × Average mean velocity = Flow volume
This technique is applied over each frame of the cardiac cycle, and a flow volume curve is generated to represent flow over time during the cardiac cycle. Forward flow is depicted as flow above zero, whereas regurgitant flow is shown below the baseline ( Fig. 35-17 ). Calculating the area outlined by the flow curve below the baseline yields the volume of regurgitation, and the area outlined by the curve above the baseline gives the volume of forward flow. Flow volume curves accurately determine the regurgitant fraction and have high interstudy reproducibility in patients with aortic or pulmonic regurgitation ( Table 35-3 ).

| Regurgitant Volume (mL) | Regurgitant Fraction (%) | |
|---|---|---|
| Mild | <30 | <30 |
| Moderate | 30-59 | 30-49 |
| Severe | >60 | >50 |
Mitral regurgitation and tricuspid regurgitation are evaluated using slightly different methods because of the configuration of the valves and the multiple angles of the regurgitant flow. If only one valve is regurgitant, the difference between the right and left ventricular stroke volume calculated volumetrically with stacked short-axis images is equal to the volume of regurgitation. A useful approach is to check this value internally with VEC phase contrast MRI as described later.
Two different methods using phase contrast MRI are available, and they are similar for the evaluation of mitral or tricuspid regurgitation; the aorta and left ventricle are assessed for mitral regurgitation, and the pulmonary artery and right ventricle are evaluated for tricuspid regurgitation. These methods hold in the absence of coexisting aortic or pulmonic regurgitation.
First Method
- 1.
An ROI is drawn around the proximal ascending aorta on the magnitude and phase VEC MRI images to determine forward flow.
- 2.
Left ventricular stroke volume (end-diastolic volume minus end-systolic volume [EDV – ESV]) obtained from volumetric measurements is calculated.
- 3.
Mitral regurgitant volume = Left ventricular stroke volume – Ascending aorta forward flow.
- 4.
Mitral regurgitant fraction = Mitral regurgitant volume / Left ventricular stroke volume.
Second Method
- 1.
An ROI is drawn around the mitral annulus to measure forward flow during diastole.
- 2.
An ROI is drawn around the ascending aorta to determine forward flow during systole.
- 3.
Mitral regurgitant volume = Forward flow mitral valve – Forward flow aorta.
- 4.
Mitral regurgitant fraction = Mitral regurgitant volume/Left ventricular stroke volume ( Table 35-4 ).
Table 35-4
Grading Mitral Regurgitation
Regurgitant Volume (mL)
Regurgitant Fraction (%)
Mild
<30
<30
Moderate
30-59
30-49
Severe
>60
>50
Clinical Pearl
A flow volume curve is derived from VEC phase contrast MRI and is useful for determining regurgitant fraction.
Cardiovascular Effects of Increased Volume
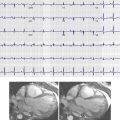
Ventricular mass and function are reliably documented using the cine SSFP technique. Contiguous short-axis images are acquired from the cardiac apex to the base of the heart in multiple phases of the cardiac cycle. When images are viewed as a cine loop, the examiner can assess global ventricular function and regional wall motion.
ESV and EDV are calculated using multislice short-axis images. ROIs are drawn around the ventricular cavity at end-systole and end-diastole and are multiplied by slice thickness and interslice gap to obtain ESV and EDV, respectively. ESV and EDV are used to calculate the EF ( Fig. 35-18 ):
EF = ( EDV − ESV ) / EDV
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree