(1)
DIBIMEF, Department of Radiology, University Hospital “P. Giaccone”, Palermo, Italy
(2)
Department of Radiology, University Hospital Catania, Catania, Italy
12.1 Introduction
12.3.1 Pathophysiology
12.3.2 Echocardiography
12.4.1 Technique
12.4.2 Applications
12.5 Computed Tomography
12.5.1 Technique
12.5.2 Applications
Abstract
Valvular heart disease (VHD) refers to a wide spectrum of cardiac disorders that affect a large number of patients. Echocardiography is still considered the pivotal imaging method to evaluate the cardiac valves. However, echocardiography has some intrinsic limitations due to operator dependence and patient habitus. In the last two decades three-dimensional techniques such as magnetic resonance imaging (MRI) and computed tomography (CT) have provided valuable assessment of VHD. MRI overcomes the limitation of the poor acoustic window of echocardiography without ionizing radiation. Nowadays, MRI aims to be considered as the reference standard for imaging cardiac valves because of the improved morphological visualization and reproducible assessment of ventricular volume, mass, and function. CT can display the morphologic features of valves and allow their functional assessment in the setting of congenital and acquired disorders. Moreover, CT angiography may be performed to rule out coronary artery disease in presurgical planning and to follow up patients with prosthetic valves.
Abbreviations
AR
Aortic regurgitation
AS
Aortic stenosis
CAD
Coronary artery disease
CMR
Cardiac magnetic resonance
CT
Computed tomography
CTCA
Computed tomography coronary angiography
ECG
Electrocardiography
GE
Gradient echo
MR
Mitral regurgitation
MRI
Magnetic resonance imaging
MS
Mitral stenosis
PC
Phase contrast
SSFP
Steady-state free precession
TE
Time of echo
TEE
Transesophageal echocardiography
TI
Time of inversion
TR
Tricuspid regurgitation
TS
Tricuspid stenosis
TTE
Transthoracic echocardiography
VENC
Velocity encoding value
VHD
Valvular heart disease
12.1 Introduction
Valvular heart disease (VHD) is responsible for significant mortality and morbidity worldwide. An estimated 23,313 patients died from VHD in the United States in 2007, with aortic and mitral valve disease accounting for 15,183 and 2,644 deaths, respectively. In 2007, an estimated 98,000 patients were discharged with a diagnosis of VHD (Roger et al. 2011). According to echocardiographic data, the prevalence of VHD increases with age from 0.7 % in adults from 18 to 44 years to 13.3 % in individuals over 75 years (Nkomo et al. 2006). Cardiac valves are composed of fibromuscular tissue, which may be affected by age-related changes such as myxomatous degeneration, collagen accumulation, sclerosis, and calcification. Common aging phenomena include calcification of the aortic and mitral leaflets and annulus. Aortic stenosis is considered the most frequent indication for valve replacement in elderly individuals (Vahanian et al. 2007; Bonow et al. 2006). Coronary atherosclerosis and aortic calcifications are directly correlated in elderly patients with an increased risk of major cardiac events (Pohle et al. 2001). Hypertension or ischemic heart disease may cause mitral valve regurgitation, while pulmonary hypertension and heart failure can lead to tricuspid or pulmonic regurgitation. Elderly patients may undergo valve replacement with an increased risk of infection and complications (Dauterman et al. 2003).
Echocardiography has been widely deployed for the evaluation of VHD because of its accessibility and cost-effectiveness (Vahanian et al. 2007; Bonow et al. 2006). Three-dimensional techniques such as magnetic resonance imaging (MRI) and computed tomography (CT) have been implemented recently in VHD imaging. MRI could evolve into becoming a reference standard because of its high reproducibility and lack of ionizing radiation exposure. Despite the exposure to radiation, CT may be useful in preoperative planning of VHD to exclude coronary artery disease (CAD) and in the follow-up of valve replacement. This chapter provides the basis of VHD pathophysiology in the elderly with reference to echocardiographic diagnostic criteria and finally focuses on the most recent clinical applications of MRI and CT in this setting.
12.2 Anatomy of the Cardiac Valves
The heart is a hollow organ divided into four chambers, two atria and two ventricles, coupled into two atrioventricular pumps (right and left heart) independent of each other and separated by interatrial and interventricular septa. The heart is a pump that circulates the blood in the vessels by the pulmonary and systemic circulations. The superior and inferior vena cava lead the systemic venous blood to the right atrium and from here via the tricuspid valve to the right ventricle. Systolic contraction pumps the blood via the pulmonary valve into the pulmonary artery and pulmonary circle. Oxygenated blood is conveyed to the pulmonary veins (usually four, often with variable number and branching), which drain into the left atrium and from here via the mitral valve into the left ventricle. Then, systolic contraction pumps the blood through the aortic valve into the systemic circulation.
Therefore, cardiac valves regulate the unidirectional flow between the cardiac chambers and the great vessels. The tricuspid and mitral valves regulate the atrioventricular flow. The tricuspid is the three-flapped (composed of septal, inferior, and anterosuperior leaflets) valve of the right section of the heart, while the mitral is the two-flapped (composed of aortic and mural leaflets) valve of the left section. The mitral valve area ranges from 4 to 6 cm2. In the longitudinal cardiac axis, the tricuspid valve is always positioned more cranially to the mitral valve. Chordae tendineae (thickness 0.4–1.2 mm for the mitral valve) connect the leaflets to the papillary muscles of the ventricles, preventing valve prolapse and backflow. The apparatus of the tricuspid incorporates three papillary muscles: anterior (the largest), medial, and inferior. The apparatus of the mitral valve includes two paired papillary muscles incorporated in the anterolateral and posteromedial walls of the left ventricle (Ranganathan et al. 1970).
The pulmonic and aortic valves are called semilunar because of their crescent shape. The pulmonary valve is placed at the junction of the right ventricle and pulmonary artery; the aortic valve is positioned at the junction of the left ventricle and aorta. Both valves lack chordae tendineae. The semilunar valves are made up of three leaflets which open as pressure rises in the ventricles and close as pressure increases in the pulmonary artery and aorta, preventing backflow of blood from the great arteries into the ventricles. The pulmonary artery arises from the outflow tract of the right ventricle via the pulmonary valve. The infundibular muscles separate the leaflets of the pulmonic and tricuspid valves. The aortic valve presents usually three leaflets with corresponding sinuses, commissures, and coronary artery ostia; the area of the normal aortic valve varies from 2.5 to 4 cm2 (Brickner et al. 2000). The aortic root connects the aorta to the atrioventricular fibrous annulus and myocardium. The first tract of the aorta is dilated, because of the swellings of the sinuses, and is called the bulb. There are usually three aortic sinuses (known also as the Valsalva sinuses): the right anterior sinus giving rise to the right coronary artery, the left posterior sinus giving rise to the left coronary artery, and the posterior noncoronary sinus. The junction between the aortic root containing the sinuses of Valsalva and the ascending aorta is called the sinotubular junction. The right coronary artery arises from an ostium located in the middle of the right sinus of Valsalva. The left main coronary trunk originates from the middle portion of the left sinus of Valsalva giving rise to the left anterior descending and circumflex arteries. A split origin of the left anterior descending and circumflex arteries from the left sinus of Valsalva may be observed (0.67 %), as well as a large number of coronary origin and course anomalies (Angelini et al. 2002).
The most common congenital valve anomaly is the bicuspid aortic valve which involves 0.5–1 % of the population (Basso et al. 2004) and occurs in 13.7 per 1,000 people (Hoffman and Kaplan 2002). The bicuspid valve is typically made of two unequal-sized cusps instead of three. It is often asymptomatic in childhood and misdiagnosed. However, the abnormal shear stress leads progressively to valve calcification and stenosis, or in some cases to aortic root dilation (Tzemos et al. 2008). Bicuspid aortic valve may be associated with aorta coarctation (Ciotti et al. 2006).
12.3 Valvular Heart Disease
12.3.1 Pathophysiology
Although VHD is less common in developed countries than CAD, heart failure, or hypertension, aging populations and considerable advances in diagnosis and treatment have led to a new interest in the field. The decline of acute rheumatic fever due to better prophylaxis of streptococcus infections highlights the decrease in the incidence of rheumatic valve disease, whereas increased life expectancy accounts for the raise of degenerative VHD in western countries. Nowadays, the most frequent VHD are aortic stenosis (AS) and mitral regurgitation (MR), whereas aortic regurgitation (AR) and mitral stenosis (MS) have become less common. However, rheumatic heart disease is still present in developed countries because of immigration and sequelae of rheumatic fever in elderly patients. The treatment for severe and acute VHD is valve replacement with prosthetic devices by means of conservative surgical approaches or with new percutaneous interventional techniques.
12.3.1.1 Mitral Stenosis
The major cause of MS is rheumatic fever, which affects women more frequently and induces progressive changes of the mitral valve: inflammatory responses, such as thickening at the leaflet edges, fusion of the commissures, and chordal shortening, and scarring features, such as fibrosis, increased collagen and tissue cellularity, and finally calcification (Sagie et al. 1996). The valve may present a “fish mouth” appearance because of the symmetric fusion of the commissures resulting in a small oval orifice in diastole. At end stages the thickened leaflets may be adherent and rigid leading to combined MS and MR. Reduction of mitral valve orifice (<1.5 cm2 in moderate MS; <1 cm2 in severe MS) determines an increase in transmitral gradient, and sequentially in the left atrial, pulmonary circle, and right heart pressures. Exertional dyspnea may be precipitated by tachycardia and atrial fibrillation in elderly patients and trigger loop mechanisms of left atrial pressure elevation (Fuster et al. 2006). Left atrial enlargement is associated with an increased risk of thrombus formation and systemic embolism. A number of conditions in the elderly may simulate the pathophysiology of MS: extensive mitral annular calcification, thrombus or myxoma in the left atrium, and large vegetations of infective endocarditis.
12.3.1.2 Mitral Regurgitation
Abnormalities in any component of the mitral valve apparatus may lead to MR. The most common causes of organic MR include mitral valve prolapse, rheumatic heart disease, infective endocarditis, ischemic heart disease (with papillary muscle rupture), and collagen vascular disease. MR may be also functional and secondary to dilation of the left ventricle. MR may be acute in case of chordae tendineae or papillary muscle rupture, or infective endocarditis. Mitral valve prolapse (1–2 % of the population) refers to a systolic billowing of mitral leaflets into the left atrium, which is usually asymptomatic, but may lead to severe MR (Freed et al. 2002). In severe MR, the unprepared left cardiac chambers cannot accommodate the regurgitant volume, which causes pulmonary congestion and edema. In these patients valve repair or replacement must often be performed urgently. Patients with mild to moderate MR may remain asymptomatic for many years; however, MR tends to develop over time with a slightly increase in volume overload due to an enlargement of the effective orifice area (Enriquez-Sarano et al. 1999). Eccentric left ventricle hypertrophy and dilation may consequently compensate the increase in volume overload.
12.3.1.3 Aortic Stenosis
AS has become the most frequent type of VHD in Europe and North America, primarily presenting as degenerative calcific stenosis in the elderly. Defined as an irregular valve thickening without obstruction to left ventricular outflow, AS is present in about 25 % of adults over 65 years. AS is widely associated with cardiovascular risk factors (Stewart et al. 1997) and adverse clinical outcome (Otto et al. 1999). The left ventricle gradually adapts to the systolic pressure overload through concentric hypertrophy. However, if the hypertrophic process is inadequate and relative wall thickness does not increase in proportion to pressure, wall stress increases and the high afterload causes a decrease in ejection fraction (Krayenbuehl et al. 1988). Unfortunately, the hypertrophic heart may have reduced coronary perfusion, even in the absence of CAD (Bache et al. 1981). Then, exercise or tachycardia can produce a misdistribution of coronary blood flow and subendocardial ischemia, which can worsen left ventricular dysfunction. Another issue that is particularly common in elderly patients, especially in women, is the inappropriate degree of hypertrophy associated with high perioperative morbidity and mortality (Aurigemma et al. 1994).
12.3.1.4 Aortic Regurgitation
Etiology of AR includes idiopathic dilation of the aorta, congenital abnormalities (bicuspid valves), calcific degeneration, rheumatic disease, infective endocarditis, systemic hypertension, myxomatous degeneration, dissection of the ascending aorta, and Marfan syndrome. AR may be acute (infective endocarditis, aortic dissection, and trauma) or more often chronic. Dilation of the aortic root determines tension and bowing of the individual cusps, which may thicken, retract, and become too short to close the orifice. AR secondary to marked dilation of the ascending aorta is now more common than primary valve disease in patients undergoing valve replacement (Gelfand et al. 2006). The left ventricle responds to the overload of chronic AR with a series of compensatory mechanisms, including an increase in end-diastolic volume and chamber compliance, and a combination of eccentric and concentric hypertrophy. In a large subset of patients, the balance between afterload excess, preload reserve, and hypertrophy cannot be accommodated indefinitely, so that the reduction of ejection fraction leads to dyspnea, and the diminished coronary flow reserve leads to exertional angina and myocardial ischemia (Nitenberg et al. 1988). In acute severe AR, compensatory mechanisms are not efficient to accommodate the volume overload and patients present with pulmonary edema or cardiogenic shock.
12.3.1.5 Right-Sided Valve Disease
Tricuspid stenosis (TS), which is almost exclusively of rheumatic origin, is rarely observed. It is almost always associated with left-sided valve lesions which dominate the presentation. A relatively modest diastolic pressure gradient (>5 mmHg) is usually sufficient to elevate right atrial pressure to levels that result in systemic venous congestion. Tricuspid regurgitation (TR) is more often a functional rather than a primary lesion, depending on the annular dilation secondary to right ventricular pressure and/or volume overload associated with pulmonary hypertension and left-sided VHD. TR can also occur secondary to right ventricle infarction (Rivera et al. 1993).
Pulmonary valve stenosis is often congenital, while regurgitation may occur secondary to pulmonary hypertension and dilation of the pulmonary artery in connective tissue disorders.
12.3.2 Echocardiography
Echocardiography still remains the reference standard to evaluate valve structure and function (Figs. 12.1 and 12.2). It has been widely deployed for the assessment of VHD because of its high accessibility and cost-effectiveness (Vahanian et al. 2007; Bonow et al. 2006). The main limitations of echocardiography are operator dependence and poor acoustic window in obese patients.
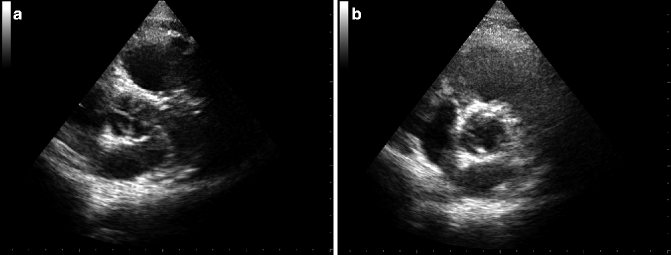
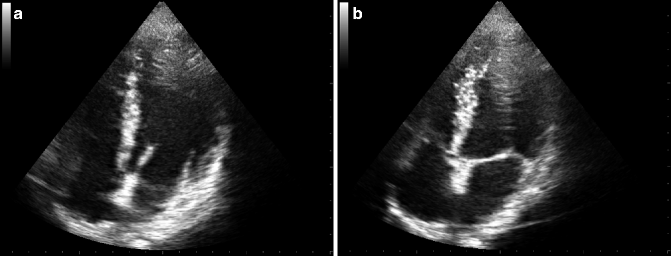

Fig. 12.1
TTE images of aortic valve in diastolic (a) and systolic (b) phase

Fig. 12.2
TTE images of mitral valve in diastolic (a) and systolic (b) phase
12.3.2.1 Mitral Stenosis
Transthoracic echocardiography (TTE) is the main method to assess the severity and consequences of MS. Severity of MS should be quantified using two-dimensional planimetry and the pressure half-time method, which are complementary approaches for measuring valve area (Table 12.1) (Bonow et al. 2008). Echocardiography may also evaluate pulmonary artery pressures, the presence of associated MR, and the size of the left atrium. Transesophageal examination (TEE) should be performed to exclude left atrial thrombosis before percutaneous mitral commissurotomy, or after an embolic episode, or when TTE provides suboptimal information. Stress tests may be helpful in patients with unclear symptoms.
Table 12.1
Echocardiographic criteria of mitral stenosis severity
Features | Mild | Moderate | Severe |
|---|---|---|---|
Mean gradient (mmHg) | <5 | 5–10 | >10 |
Pulmonary artery systolic pressure (mmHg) | <30 | 30–50 | >50 |
Valve area (cm2) | >1.5 | 1.0–1.5 | <1.0 |
12.3.2.2 Mitral Regurgitation
Echocardiography is the pivotal examination and must include the assessment of severity and consequences of MR. Several methods can be used to determine the severity of MR (Table 12.2) (Bonow et al. 2008). TTE may reveal a central color flow jet in a structurally normal apparatus suggesting annular dilation from left ventricle dilation or tethering of the posterior leaflet, because of regional dysfunction in patients with ischemic heart disease. TTE may display eccentric flow jet of MR with abnormalities of the valve apparatus (calcifications, thickening, and redundancy of leaflets) indicating organic MR. Color flow mapping of regurgitant jets is the easiest diagnostic method, but its accuracy is limited. Quantitative methods to measure regurgitant fraction, regurgitant volume, and effective regurgitant orifice area are more accurate. In the hemodynamically stable patient, if CAD is suspected, conventional coronary angiography should be performed to plan a potential myocardial revascularization.
Table 12.2
Echocardiographic criteria of mitral regurgitation severity
Features | Mild | Moderate | Severe |
|---|---|---|---|
Color Doppler jet area | <4 cm2 | Greater than mild | >40 % of LA |
<20 % LA area | No criteria for severe MR | Swirling in LA | |
Doppler vena contracta width (cm) | <0.3 | 0.3–0.69 | ≥ 0.70 |
Regurgitant volume (ml/beat) | <30 | 30–59 | ≥60 |
Regurgitant fraction (%) | <30 | 30–49 | ≥50 |
Regurgitant orifice area (cm2) | <0.20 | 0.2–0.39 | ≥0.40 |
Left atrial size | Enlarged | ||
Left ventricular size | Enlarged |
12.3.2.3 Aortic Stenosis
TTE is useful for evaluation of aortic valve anatomy and function and for determining the left ventricle response to pressure overload. Severity of AS can be easily defined with Doppler echocardiographic measurements of aortic jet velocity, mean transvalvular pressure gradient, and valve area (Table 12.3) (Cheitlin et al. 2003; Bonow et al. 2008). Stress echocardiography using low-dose dobutamine may be helpful to distinguish truly severe AS from the rare case of pseudo-severe AS, in which patients present a functional small valve area. Severe AS shows only small changes in valve area (<0.2 cm2) with increasing flow rate but significant increase in gradients (>50 mmHg). TEE is needed when valve planimetry of TTE is inadequate.
Table 12.3
Echocardiographic criteria of aortic stenosis severity
Features | Mild | Moderate | Severe |
|---|---|---|---|
Jet velocity (m/s) | <3.0 | 3.0–4.0 | >4.0 |
Mean gradient (mmHg) | <25 | 25–40 | >40 |
Valve area (cm2) | >1.5 | 1.0–1.5 | <1.0 |
Valve area index (cm2/m2) | <0.6 |
12.3.2.4 Aortic Regurgitation
Echocardiography may identify the cause of AR by demonstrating bicuspid valve, thickening of the cusps, prolapse, flail leaflet, or vegetations. TTE is useful for the measurement of left ventricle end-diastolic and end-systolic dimensions and volumes, ejection fraction, and mass. In addition, the size and shape of the aortic root can be evaluated, although visualization of the ascending aorta is not always adequate and may require additional imaging procedures such as TEE, CT, or MRI. Doppler echocardiography and color flow Doppler imaging are the most accurate techniques in the evaluation of AR (Table 12.4) (Bonow et al. 2008).
Table 12.4
Echocardiographic criteria of aortic regurgitation severity
Features | Mild | Moderate | Severe |
|---|---|---|---|
Color Doppler jet width | <25 % of LVOT | Greater than mild; no criteria for severe AR | >65 % of LVOT |
Doppler vena contracta width (cm) | <0.3 | 0.3–0.6 | >0.6 |
Regurgitant volume (ml/beat) | <30 | 30–59 | ≥60 |
Regurgitant fraction (%) | <30 | 30–49 | ≥50 |
Regurgitant orifice area (cm2) | <0.10 | 0.10–0.29 | ≥0.30 |
Left ventricular size | Increased |
12.3.2.5 Right-Sided Valve Disease
TTE may assess tricuspid valve structure and motion (severe TS: valve area <1 cm2). Doppler echocardiography permits estimation of the severity of TR (severe TR: vena contracta width >0.7 cm, systolic backflow in hepatic veins) (Bonow et al. 2008). TTE may also show abnormal motion of the septum which is characteristic of right ventricle volume overload in diastole and/or septal flutter. Abnormal Doppler signals in the right ventricle outflow tract are observed in patients with severe pulmonic stenosis (jet velocity >4 m/s; gradient >60 mmHg) or with pulmonic regurgitation secondary to pulmonary hypertension (color jet; continuous signal with a sharp deceleration slope) (Bonow et al. 2008).
12.4 Magnetic Resonance Imaging
12.4.1 Technique
Doppler echocardiography with color flow mapping has considerably affected the diagnostic work-up for the evaluation of VHD. However, TTE may not be conclusive in patients with poor acoustic windows; it is operator dependent and relies on several geometric assumptions particularly for the quantification of regurgitant lesions and the measurement of left ventricular systolic function. Invasive techniques such as cardiac catheterization may be employed in the preoperative evaluation of the coronary arteries, quantification of cardiac hemodynamic, or investigation of discrepancies between symptoms and echocardiography data. Several papers have demonstrated that MRI may be a promising alternative or complement to TTE (Globits and Higgins 1995). Noninvasive MRI supplies three-dimensional anatomy and functional assessment, with a potentially more accurate measurement of ventricular function than echocardiography (Higgins and Sakuma 1996).
The most widely used cine pulse sequence on MRI to assess valve anatomy and ventricle function is the balanced steady-state free precession (b-SSFP) sequence (Fig. 12.3). High contrast between blood pool and surrounding structures is achieved with excellent spatial (in-plane resolution of 1.4 mm) and temporal resolution (∼35 ms). SSFP sequences have several monikers, such as TrueFISP (Siemens), balanced FFE (Philips), and FIESTA (General Electric) (Karamitsos and Myerson 2011




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree