27
Varicocele and Female Infertility
Since the beginning of recorded history, humans have placed an emphasis on fertility. In many cultures, childlessness is viewed as a deficiency on the part of both male and female partners. Even in today’s modern society, procreation is considered a basic human right, and infertile couples must cope with difficult psychological and social problems. Unfortunately, the incidence of infertility in newly married couples is on the rise. In industrialized countries, the incidence of infertility has increased from 7 to 8% in 1960 up to 20 to 35% today.1 In response to the growing problem, new medical therapies have been advanced both to diagnose and to treat the wide variety of disorders that can cause infertility. Over the past two decades, advances in fluoroscopically guided catheter techniques have helped to treat some of these disorders. This chapter reviews these techniques as they pertain to the treatment of varicoceles, fallopian tube occlusions, and cervical stenosis.
 Varicoceles
Varicoceles
Varicocele, defined as abnormal distention of veins in the pampiniform plexus, has long been recognized to be associated with testicular dysfunction (Fig. 27-1). As early as the first century AD, Celsus described the association between swollen veins over the testes and testicular atrophy.2 The realization that varicocele ablation can restore testicular function was made in the late 1800s when Bar-well described the restoration of testicular function with subsequent conception after varicocele occlusion.3 Although the association with testicular dysfunction was recognized, treatment was primarily directed toward the relief of painful symptoms. The procedure was not used to repair varicoceles in infertile males. It was not until 1952, when Tuloch reported the restoration of fertility in an azospermic male, that varicocele ligation as a treatment for infertility gained widespread acceptance.4 Since then, varicocelectomy has become the most common operation performed for male infertility.5
The importance of the varicocele and its role in infertility lies in its common occurrence in the general population. Among young men, the reported incidence of varicoceles in the literature ranges from 5 to 26% with a mean incidence of approximately 15%.6–11The incidence among infertile men is much higher, occurring in approximately 40% of patients.12,13
Treatment for this disorder has focused on the ligation of the spermatic vein, and various surgical techniques have been described in the literature.14–17 In 1977, Iaccarino described a percutaneous technique for radiologically sclerosing the spermatic vein.18 Since its introduction, technical enhancement and the development of newer embolic agents has made this a safe, effective, and relatively simple procedure.
Anatomy
The veins of the spermatic cord emerge from the mediastinum of the testicle to form the pampiniform plexus, which consists of three groups of freely anastomosing veins: the anterior, middle, and posterior. The external spermatic vein and the cremasteric veins constitute the posterior group. These veins course posterior to the spermatic cord and drain into the inferior epigastric veins at the level of the external inguinal ring. The middle group courses medially along the vas deferens and drains into the internal iliac veins. The anterior group, or the internal spermatic vein (ISV), accompanies the spermatic artery as it courses through the retroperitonium. On the left side, the ISV drains into the left renal vein. On the right, the ISV usually enters the inferior vena cava (IVC) just below the origin of the right renal vein (Fig. 27-2). Drainage of the right ISV into the right renal vein has been noted in 10% of cases.19 In most instances, the ISV contains valves that are normally located within one centimeter of the vein opening.20
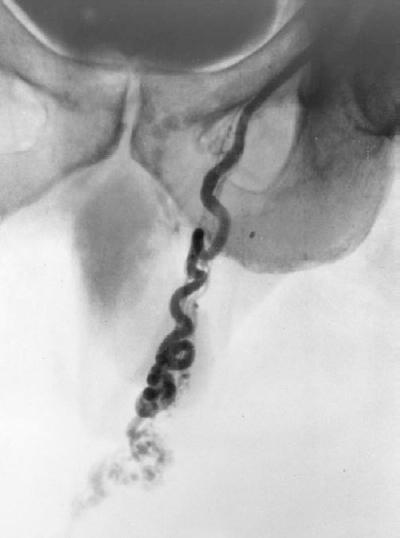
FIGURE 27-1. Internal spermatic vein (ISV) venogram demonstrating the reflux of contrast material into the dilated veins of a left varicocele. This image normally should not be obtained because the scrotum should be shielded from radiation exposure during venography.
The anatomy of the ISV is highly variable, and multiple collateral pathways exist. Knowledge of these pathways offers insight into the pathogenesis of varicoceles and is important in providing effective treatment.21,22 Several collateral pathways anastomose with the upper portion of the ISV. These pathways include hilar, capsular, intrarenal, retroperitoneal and colonic communications (see Fig. 27-2). Hilar collaterals, which emanate from the hilar portion of the renal vein and anastomose with the
ISV, are present in 14% of patients (Fig. 27-3).23 Capsular collaterals arise from intrarenal or capsular veins and pursue a tortuous course before joining the ISV. Retroperitoneal collaterals, which are present in 40% of patients, provide communications between the upper ISV and lumbar veins (Fig. 27-4).24 Communications occur frequently between the colic veins, the inferior mesenteric vein on the left, and the superior mesenteric vein on the right.24 These vessels usually contain valves and join with the ISV at the level of the iliac crest. Bridging collaterals, draining directly into the IVC, also occur but involve the right ISV far more often than the left.24 All these collateral pathways, when present, can permit venous reflux by bypassing competent ISV valves.
Collaterals that anastomose with the lower portion of the ISV include parallel channels and venous communications to the internal iliac and inferior epigastric veins. Parallel channels are fine, multiple, threadlike veins that originate and terminate in the ISV (Fig. 27-5). If left unoccluded, these seemingly insignificant vessels can, in time, enlarge and reconstitute the ISV. Collaterals to the internal iliac and inferior epigastric veins are infrequent; for example, internal iliac vein anastomoses were found to be present in only 2% of cases.24
Etiology
Varicoceles can be primary or secondary. Secondary varicoceles usually occur in older patients and result from spermatic or renal vein obstruction by tumor or massive hydronephrosis.25,26 Primary varicocele, by far the more common type, is most prevalent in adolescent males and occurs on the left side in approximately 90% of cases.27 It is bilateral in 8 to 9% and is right-sided in 1 to 2%.28 Several hypotheses have been advanced to explain this asymmetric presentation. Incomplete or absent valves, which occur more frequently on the left side, have been cited as one possible explanation (Fig. 27-6). Ahlberg et al. found absent valves in 40% of left spermatic veins examined at autopsy compared with 23% on the right.19 Unfortunately, the frequent occurrence of valvular abnormalities is inconsistent with the relatively low 15% incidence of varicoceles in the general population. Additionally, several investigators have noted varicoceles in patients with competent ISV valves.29 Other hypotheses, such as the vertical course and perpendicular insertion of the left spermatic vein30 and the nutcracker phenomenon, have been proposed. The nutcracker phenomenon is compression of the left renal vein between the aorta and the superior mesenteric artery in the upright position.31–33 The physiologic obstruction of the renal vein leads to increased hydrostatic pressure in the left ISV and formation of the varicocele. More recently, a developmental etiology has been advanced. The embryogenesis of the left venous system is more complex than the right, and developmental anomalies are common. Disordered involution of the cardinal veins during development results in persistent intercardinal vein anastomoses. These collaterals permit retrograde flow in the ISV and varicocele formation.29
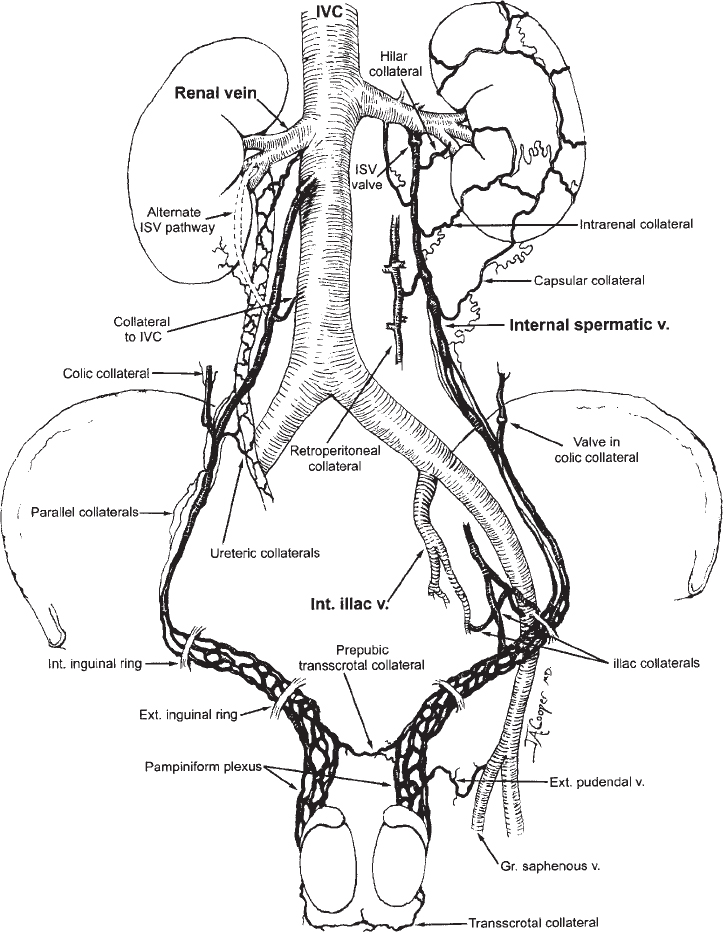
FIGURE 27-2. Venous anatomy of the internal spermatic veins.
Pathophysiology
There is great debate about whether and how varicoceles effect spermatogenesis. Some investigators claim that the relationship, if any, is coincidental.34 Others have demonstrated significant alterations in sperm density motility and morphology in association with varicoceles.35, 36 How the varicocele, which is predominantly a unilateral process, causes bilateral testicular dysfunction is obscure. Several hypotheses, including scrotal hyperthermia,37 retrograde flow of toxic metabolite such as prostaglandin’s E2 and F2α38,39 and hypoxia due to venous stasis, have been advanced.40 It is possible that many of these factors act in conjunction to cause impaired spermatogenesis. This damage, once begun, progresses over time and results in testicular atrophy and irreversible cellular damage.
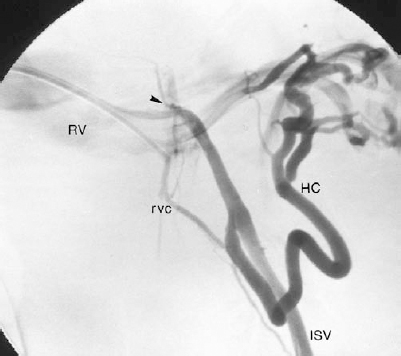
FIGURE 27-3. Left internal spermatic vein (ISV) venogram demonstrating an atypical origin of the ISV from the superior aspect of the left renal vein (arrowhead) as well as a large hilar and smaller renal vein (RV) collateral. RVC, renal vein collateral; HC, hilar collateral.
Diagnosis
Varicoceles are diagnosed most often on clinical examination and are described by their size. A grade 1 varicocele is small and can be palpated only while the patient performs a valsalva maneuver. Grade 2 varicoceles are easily palpable but are not visible, whereas grade 3 varicoceles can be detected by visual scrutiny alone.41 Vari-coceles that cannot be detected by careful clinical examination have been termed subclinical varicoceles, which are defined as reflux in the ISV without palpable distention of the pampiniform plexus.42 The significance of the subclinical varicocele, as it relates to infertility, is a subject of ongoing debate. A recent review by Marshman et al. discovered that most investigators believe this type of varicocele can cause infertility and they argue in the favor of treatment.43 These arguments are based on observed improvement in fertility after subclinical varicocelectomy44 and the lack of correlation between varicocele size and degree of infertility.45,46
Several noninvasive diagnostic modalities are used to detect subclinical varicoceles; including thermography,47 Doppler ultrasound,48 radionuclide imaging,49 and realtime ultrasound,46 which have varying degrees of sensitivity and specificity.50,51 At present, real-time scrotal ultrasound is the preferred noninvasive imaging modality used to detect subclinical venous dilatation and reflux. The presence of a varicocele is confirmed by the visualization of two to three dilated venous channels with diameters larger than 3 mm that increase in size in the erect position or with a valsalva maneuver.46 Using these criteria, Hamm et al. reported a sensitivity of 92.2% and a specificity of 100%.52
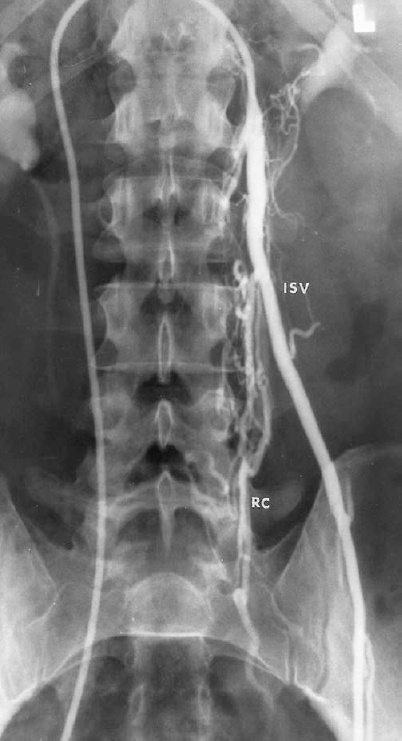
FIGURE 27-4. Left internal spermatic vein (ISV) venogram demonstrating multiple retroperitoneal collaterals (RC).
Spermatic venography was considered the most accurate means by which venous reflux could be demonstrated.19 Recently, the value of venography has been questioned. Netto et al. demonstrated reflux in normal subjects and showed no correlation between the presence of reflux and alteration in spermatogenesis.53 A criticism of this study is that the venograms were performed with the catheter tip in the spermatic vein orifice, which can bypass a proximal valve and artificially demonstrates reflux. Considering the controversial value of venography and its invasive nature, this procedure should be reserved for the determination of venous anatomy just prior to percutaneous occlusion.
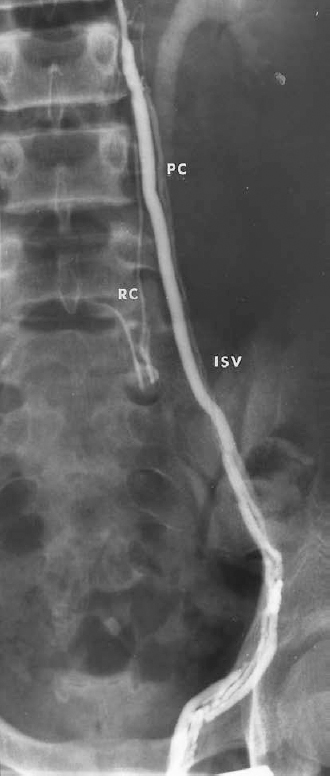
FIGURE 27-5. Left spermatic venogram demonstrating multiple threadlike parallel veins as well as a small retroperitoneal collateral (RC). IVC, internal spermatic vein; PC, parallel collaterals.
Treatment
Indications
Indications for therapeutic varicocele occlusion include pain, disfigurement, male-factor infertility, and, in the adolescent, early evidence of testicular dysfunction. Although discomfort and disfigurement are straightforward indications for treatment, infertility is not. In the infertile couple, the presence of a varicocele in the male partner, although known to cause progressive deterioration in spermatogenesis, is not indicative of oligospermia. In fact, a majority of patients with varicoceles have sperm counts well above the accepted lower limits of normal. These limits, established by the World Health Organization (WHO), are 20 million sperm per milliliter, with a progressive motility of 40 to 60%.54 Hargreave reported a 20% incidence of varicoceles among the men seen in his infertility clinic: however, only 6.4% had a varicocele associated with sperm densities lower than 20 million per milliliter.2 To confuse the issue further, many men with oligospermia have been able to father children. In 1993 Hargreave etal. reported that in the absence of other factors, the future chances of pregnancy decrease only when the motile sperm concentration (percent motility × sperm density) falls below 2 million per milliliter, which is well below the WHO lower limits of normal.2 In this same study, an inverse relationship was shown to exist between the duration of involuntary infertility and the likelihood of future pregnancy. Thus, the longer a couple has been trying, the less likely they are to conceive without therapeutic intervention. Taking these issues into consideration, our current indication for therapeutic varicocele occlusion as a treatment for male infertility is the lack of conception after 12 months of unprotected intercourse in association with a sperm motile density below WHO lower limits.
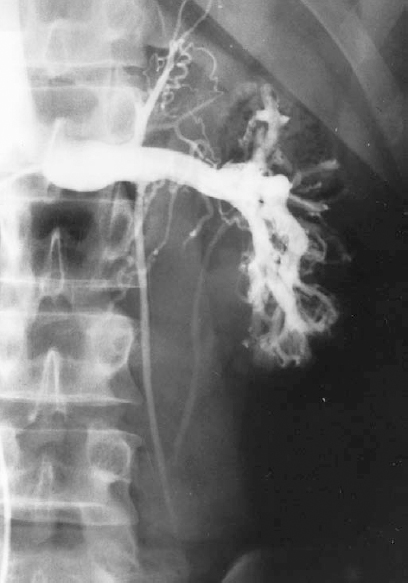
FIGURE 27-6. Left renal venogram demonstrating an incompetent proximal internal spermatic vein (ISV) valve with retrograde filling of the ISV. RV, renal vein.
In the adolescent, an asymptomatic varicocele is cause for concern because of potential future deleterious effects on fertility. The progressive, time-dependent, spermatotoxic effects of varicoceles have been demonstrated in both animal and human studies.35,55,56 There is a large difference in the incidence of palpable varicoceles between men of couples who have never been able to produce children (primary infertility) and men of couples who have produced children in the past but are currently infertile (secondary infertility).57 This difference, 35% versus 81%, respectively, strongly suggests that infertility is acquired from the presence of a long-standing varicocele. Cheval and Purcell demonstrated a time-dependent decline in sperm density and motility in a group of men with varicoceles who initially had normal semen parameters.58 The difficulty is that not all varicoceles will cause clinically relevant future testicular damage. Therapy based on the presence of a varicocele alone could result in substantial overtreatment. Therefore, specific features need to be identified that will indicate which patients are at higher risk for future infertility. The most commonly used indicators are based on sperm parameters, testicular volume measurements, and endocrine assessment.
In the older teenager, semen samples are often not difficult to obtain. An abnormal result in a teenager with a varicocele is an indication for treatment. In an adolescent in whom it is not possible to obtain a semen specimen, loss of testicular volume ipsilateral to the varicocele indicating testicular growth arrest is considered to be the primary indication for treatment.59,60 Testicular volume can be measured by careful physical examination61 or by ultrasonography.60 Typically, there should be no more than a 2-mL difference between testes. When the volume difference exceeds 3 mL, varicocele occlusion should be performed.56 In the absence of discernible testicular volume loss, the gonadotropin releasing hormone stimulation test (Gn/RH) can help to identify men who show early evidence of testicular injury. A supranormal leuteinizing hormone and follicle stimulating hormone response to intravenous Gn/RH indicates Leydig’s cell and seminiferous tubule dysfunction.56,59 This type of response, if noted in an adolescent with a varicocele, is an indication for therapy.
Surgical treatment
The surgical approach to the treatment of varicoceles can be low (inguinal) or high (retroperitoneal) ligation of the ISVs. The inguinal approach, or modified Ivanissevich procedure, involves making a small incision over the inguinal canal to expose the spermatic and cremasteric veins. These veins are dissected free of the surrounding structures (vas deferens and testicular artery) and ligated.16 The retroperitoneal approach, or modified Palomo procedure, exposes the ISV within the retroperitoneum after it exits the inguinal canal17 To accomplish this, a small abdominal incision is made at the level of the internal inguinal ring. The fibers of the external oblique faschia and internal oblique muscle are divided to expose the dilated ISVs. Once identified, the veins are ligated and divided. Recently, laparoscopic ligation of the ISVs has become popular. This technique requires three intraperitoneal entry points for placement of the laparoscope and operative instruments.62 With the laparoscope, the inner surface of the abdominal wall can be visualized, revealing either the right or left ISV coursing through the retroperitoneum just above the internal inguinal ring. To access these veins, the parietal peritoneum is incised and the veins are isolated and ligated. Proponents of this technique have demonstrated its safety and efficacy.63,64 Critics argue that the laparoscopic approach transforms a simple extraperitoneal procedure performed through a single incision with local anesthesia into an intraabdominal procedure requiring three separate incisions and general anesthesia.65,66
Percutaneous occlusion
Approach
A method for selectively catheterizing the ISV from a femoral vein approach was first described in 1976;67 just one year later, this approach was used to occlude a refluxing ISV percutaneous.18,68 Today, femoral vein catheterization is the most commonly used approach for embolization of spermatic veins.69,70 From the right common femoral vein, the left ISV can be catheterized with a 7.3 Fr Hopkins curved catheter (Cordis Corp., Miami, FL) (Fig. 27-7). This catheter is placed in the distal left renal vein and is gently pulled back to engage the orifice of the left ISV (Fig. 27-8A). The right ISV, arising from IVC, can be catheterized with a 6 or 7 Fr Simmons-shaped catheter (Fig. 27-8B). The acute angle at which the right ISV arises from the IVC makes catheterization from the femoral route more difficult. Great care must be taken to avoid inadvertent dissection of the vein orifice with the catheter tip. After engaging the ISV orifice, subselective catheterization is often required to deliver the embolic agent, which can be difficult from a femoral approach because the direction of catheter tip travelis opposite the direction of applied force. The use of a 7 or 8 Fr Hopkins curved guiding catheter permits coaxial catheterization of the ISV and remedies this problem.71
FIGURE 27-7. A7.3 Fr Hopkins curved catheter (Cook, Bloomington, IN).
The difficulties associated with the catheterization of the ISV from the femoral vein prompted other investigators to advocate an internal jugular or basilic vein approach.72–75 From the internal jugular vein, a modified 7 Fr headhunter catheter can be used to select the origin of the right or left ISV.73,74 From this route, catheter travel and applied force are in alignment, thus facilitating deep catheterization of the vein. Additionally, the recovery period required after catheterization of the internal jugular vein is somewhat shorter than the recovery period required after femoral vein catheterization.
Venography
Irrespective of the route used, once the origin of the ISV is catheterized, a venogram must be performed. This is accomplished by vigorously injecting 10 to 20 mL of contrast material into the vein orifice while the patient performs a valsalva maneuver. Alternatively, if a tilt table is available, the contrast can be injected with the patient in reverse Trendelenbergposition. The resulting images can be recorded digitally or on a series of cut-film radiographs. The purpose of the venogram is to confirm the presence of venous reflux and to delineate the highly variable anatomy of the ISV. The anatomic information helps determine which embolic agent will best provide effective occlusion. On occasion, even with a clinically obvious varicocele, a competent ISV valve is present and no reflux can be demonstrated. In this setting, anastomosing bypass channels to the distal renal vein, capsular veins, or retroperitoneal veins must be present.23,76,77 A renal venogram sometimes can help to identify these collaterals. Unfortunately, the collateral branches are often small and tortuous and will not fill from an injection into the main renal vein. Furthermore, many of the collateral branches responsible for retrograde flow in the ISV do not drain into the renal vein. To demonstrate these collaterals more reliably, a venogram should be performed after catheterizing across the competent valve. This approach not only better defines the anatomy of the ISV it also allows an embolic agent to be deposited below the insertion of these collaterals, thus permanently preventing reflux into the veins of the pampiniform plexus (Fig. 27-9).78
Embolotherapy
The goal of percutaneous therapy is to eliminate congestion in the veins of the pampiniform plexus by occluding the refluxing ISV and all its collateral tributaries. Sclerosing agents, tissue adhesives (histoacryl), stainless steel coils, and detachable balloons all have been used to accomplish this task. Of these, no single agent has proved to be clearly superior. Each agent possesses its unique advantages and disadvantages.
Sclerotherapy
Several different sclerosing agents are used for the treatment of varicoceles. Aethoxysklerol (polidocanol),79 Sotradecol (sodium, tetradecyl, sulfate),80 Varicocid (sodium, morrhuate, benzyl alcohol),81 ethanol,82 and boiling contrast83 all have proved effective. Aethoxysklerol and Varicocid are not approved by the U.S. Food and Drug Administration (FDA) and therefore not available in the United States. Sclerosants are easily delivered by directly injecting them into the proximal ISV. Effectiveness depends on the amount injected and the length of time the sclerosant remains in contact with the veins. Ideally, the entire ISV and all its parallel collaterals should be filled with sclerosant medium for several minutes. For this reason, relative stasis in the ISV should be present. Often, just catheterization of the vein orifice can cause significant stasis. With larger ISVs, stasis can be achieved by placing a proximal occlusion balloon.84 Once flow in the ISV has been halted, the sclerosant can be injected. The sclerosant, if not radiopaque, should be mixed with contrast so that its venous distribution can be monitored with fluoroscopy. The total amount injected depends on the capacitance of the ISV. An occlusion rate of 100% was accomplished when the ratio of the injected sclerosant to the calculated ISV volume was greater than 40%. To do so, an average of 24 mL of boiling contrast was used.83 With other sclerosants, such as Varicocid, only 3 to 5 mL is injected.81,85 It is important, however, to avoid overinjection and reflux of sclerosant into critical vascular beds. Proximal reflux can cause renal vein thrombosis. Distal reflux into the pampiniform plexus can result in severe thrombophlebitis, testicular atrophy, and aspermia83 this can be prevented by externally compressing the ISV against the superior pubis ramus. Compression, which can be applied with a specially designed device,86 always should be used when a sclerosant is used. Some investigators have mixed the sclerosant medium with Gel-foam particles (Upjohn, Kalamazoo, MI) or air in an effort to slow its flow in the ISV.87 This approach reduces the likelihood of reflux and increases the time the agent remains in contact with the veins.
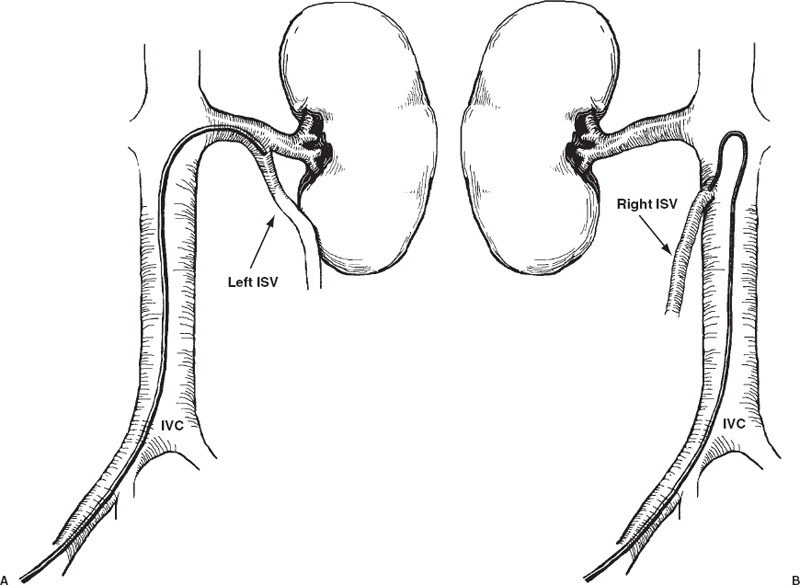
FIGURE 27-8. A: Catheters with a Hopkins curve permit catheterization of left internal spermatic vein (ISV) from the femoral vein. B: From the femoral vein, Simmons-shaped catheters can be used to select the origin of the right ISV when it arises from the inferior vena cava (IVC).
The advantages of sclerotherapy are several. For one, because the sclerosant is a fluid, it can enter and obliterate all the numerous collateral channels, thus reducing the likelihood of recurrence. Recurrence rates after sclerotherapy have been reported to range from 2 to 19%;85 however, most studies report a recurrence rate of only 2 to 3%. Another advantage is the ability to deliver the agent through small vessels that are difficult to catheterize and still effect distal occlusion. Lastly, these agents are inexpensive compared with other types of embolic agents.
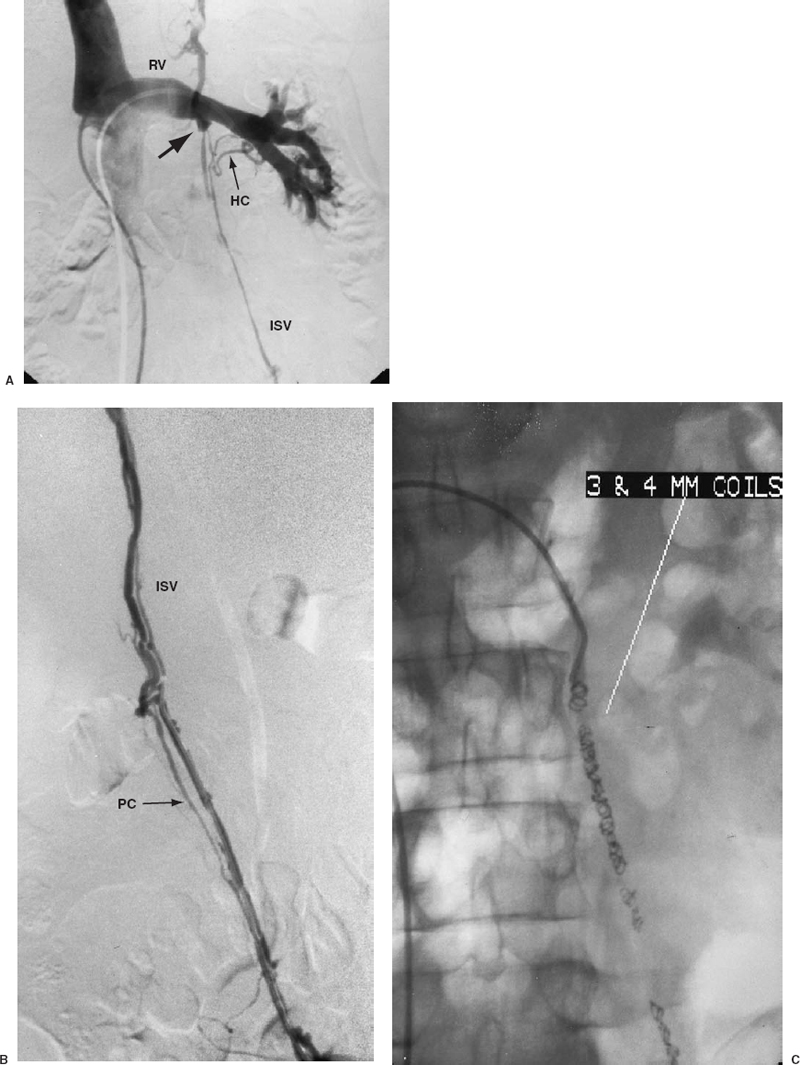
FIGURE 27-9. A: Left renal vein venogram demonstrating a competent internal spermatic vein (ISV) valve (solid arrow) with partial opacification of the ISV through hilar collaterals (HC). B: A venogram performed after selective catheterization across the proximal valve better demonstrates the venous anatomy of the ISV. Parallel collateral (PC) is noted. C: Catheterization across the valve permits distal embolization of the ISV. The entire ISV is filled with 3- and 4-mm coils to occlude all parallel collaterals and to prevent recurrence.
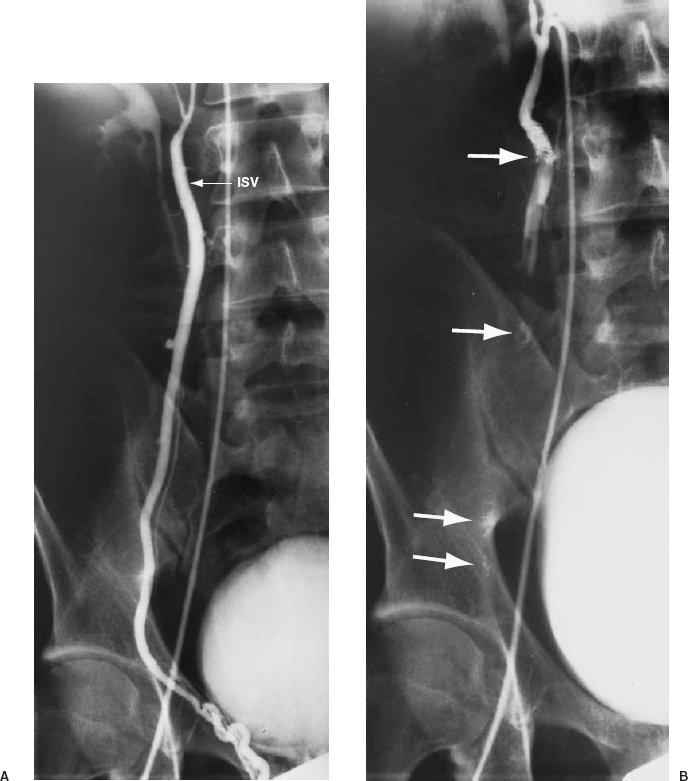
FIGURE 27-10. A: Right internal spermatic vein (ISV) venogram demonstrating reflux. B: Coils are deposited at various levels to occlude the ISV and all collaterals (solid arrows).
Disadvantages of sclerotherapy are severe pain on injection, pampiniform thrombophlebitis, and renal vein thrombosis. Injection discomfort is noted with all these agents but is most pronounced with the injection of hot contrast.83 The discomfort is short-lived, lasting from several seconds to a few minutes. Pampiniform plexitis from the sclerosant has a reported incidence of 0.5 to 1.5%.69
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree