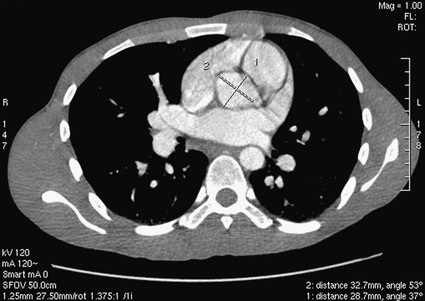
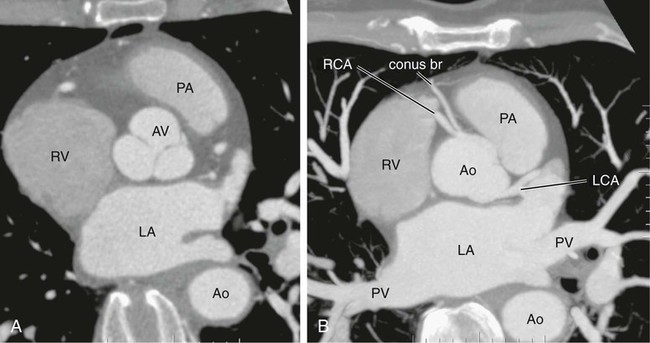
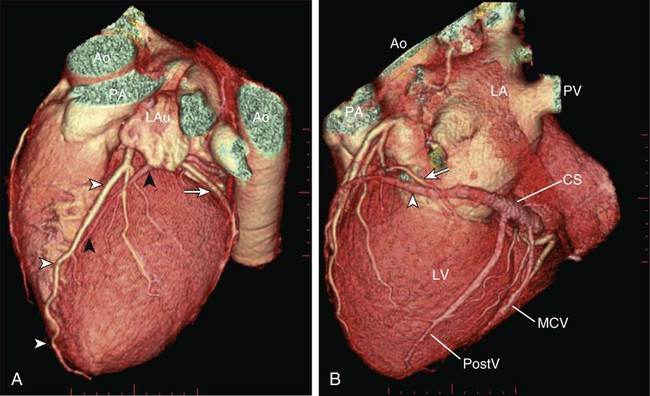
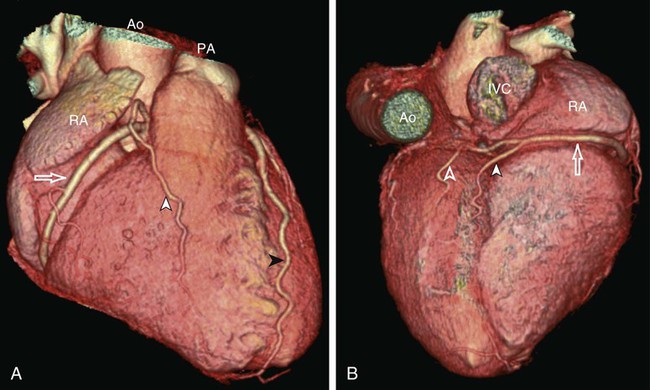
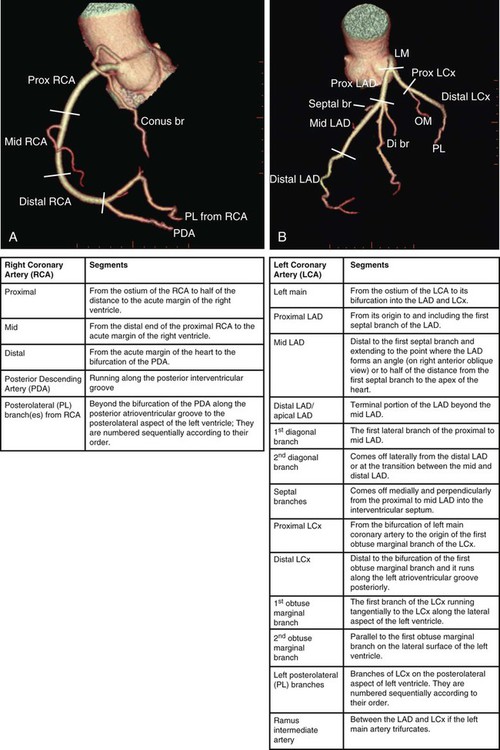
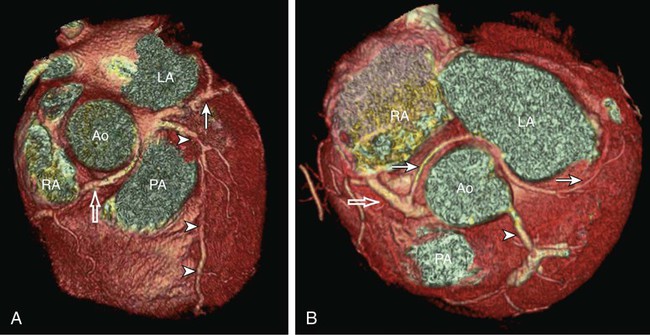
Kean H. Soon, Robert C. Heng, Kevin W. Bell, Yean L. Lim and Antony S. Walton Although the ascending aorta is classically described as being 2.5 cm in diameter and 5 cm in length, these dimensions are highly variable in practice when measured on axial computed tomography (CT) images (Fig. 82-1). Aortic diameter can exceed 30 mm at its origin in young patients with no clinical history or features of any cardiac disease. This is undoubtedly partly due to the oblique angle of measurement and pulsation artifact, but it may partly reflect the difference between an elastic living vessel filled with blood at high pressure and a rigid, empty cadaveric specimen. Dilation and elongation also occur with age and disease. The heart receives blood supply from two major epicardial arteries, the left and right coronary arteries (LCA, RCA). These two coronary arteries originate from the aortic sinuses (coronary sinuses of Valsalva). There are normally three aortic sinuses: right anterior, left posterior, and right posterior. These sinuses are bordered by three leaflets of the aortic valve that form an inverted “Mercedes sign” on cross-sectional view of the aortic root (Fig. 82-2, A). The LCA originates from the left posterior aortic sinus, whereas the RCA originates from the right anterior aortic sinus (Fig. 82-2, B). The right posterior sinus is normally free of coronary arteries. Volume-rendered three-dimensional (3D) images of CT coronary angiography are used in this section to illustrate the courses of the coronary arteries. The LMCA supplies approximately 75% of the left ventricle. Severe disease (≥70%) in the LMCA puts the patient at high risk of cardiac death. The finding of severe LMCA disease should call for immediate attention from the treating cardiologist. Severe LMCA disease is normally treated by coronary artery bypass graft (CABG) surgery. Percutaneous coronary intervention (PCI) of LMCA disease is normally reserved for those who are considered inoperable, high-risk patients, or those with protected left main disease (i.e., a patent bypass graft to the LAD or LCx). With the increasing use of drug-eluting stents in PCI, more LMCA disease is now being treated with PCI, particularly ostial or mid-LMCA disease. Distal LMCA disease that involves both the origins of the LAD and LCx is usually considered less favorable for PCI. So far, 10-year data for PCI to treat unprotected LMCA disease (i.e., the LAD or LCx is not grafted) show no significant difference in mortality rate or composite endpoint of death, myocardial infarction, or stroke between PCI and CABG. Nonetheless, PCI of the LMCA is associated with an increased risk of target-vessel revascularization despite the use of drug-eluting stents.1 Of the two main branches of the LCA, the LAD is usually the larger and more important one; it supplies the anteroseptum, anterior, anterolateral, and anteroapical walls of the left ventricle. The LAD runs along the interventricular groove on the anterior surface of the heart toward the apex of the heart (Fig. 82-3, A). It gives off medial branches known as septal branches, and lateral branches known as diagonal branches. Septal branches come off the LAD almost perpendicularly and supply the anterior two thirds of the muscular interventricular septum. The first septal branch is usually the largest, and it supplies the atrioventricular (AV [His]) bundle and proximal bundle branches. In the setting of acute anterior myocardial infarction, occlusion of the proximal LAD may be complicated by bundle branch block and hemiblock, pump failure, and ventricular tachycardia or fibrillation. The finding of severe disease in the LAD should attract prompt attention from the treating cardiologist. In patients with symptomatic hypertrophic obstructive cardiomyopathy (HOCM), transcoronary ablation of septal hypertrophy (TASH) with infusion of ethanol into septal branch(es) can be performed to reduce the septum nonsurgically.2 The LCx is usually the smaller branch of the LCA, supplying the lateral and posterior wall of the left ventricle. The LCx comes off the LMCA stem posterolaterally and runs beneath the left auricle. It runs along the left AV groove toward the posterior surface of the heart (Fig 82-3, B). The main branches of the LCx are the obtuse marginal arteries that come off tangentially to the LCx and run along the lateral aspect of the left ventricle. Beyond the edge of the left ventricle, sometimes there are more branches coming off the LCx, supplying the posterolateral aspect of the left ventricle; these are the posterolateral branches of the LCx. The RCA comes off the right anterior sinus of Valsalva and runs downwards in the right AV groove posterolaterally to the pulmonary artery and beneath the right atrial auricle (Fig. 82-4, A). The RCA gives off branches to supply the right atrium, right ventricle, posteroseptum, and to a variable degree the inferior and posterior aspects of the left ventricle. The earliest atrial branch of the RCA is normally the sinoatrial (SA) nodal artery. The SA nodal artery comes off the RCA underneath the right auricle and runs posteriorly toward the left of the superior vena caval ostium to supply the SA node. There is considerable variation in the distribution of the SA nodal artery. In about 59% of hearts, the SA nodal artery comes from the RCA. However, in about 38% of hearts, the SA nodal artery is a continuation of a large left atrial branch of the LCx. In the remaining 3%, the SA node receives dual blood supply.3 An early branch of the RCA that courses anteriorly and around the right ventricular infundibulum is the conus branch of the RCA. It may anastomose with an analogous branch from the LAD. In about 20% of hearts, the conus branch originates separately from the right anterior sinus of Valsalva instead of its parent artery, the RCA (see Fig. 82-2, A).4 As the RCA runs along the right AV groove toward the inferior border of the right ventricle, there is a branch coming off the RCA obliquely to supply the anterior surface of the right ventricle, known as the marginal branch of the RCA. Beyond the bifurcation of this marginal branch, the RCA wraps around the edge of the right ventricle and runs along the posterior AV groove to reach the crux of the heart, where the AV groove meets with the posterior interventricular groove. At the crux, the RCA gives off a branch superiorly into the interatrial septum to supply the AV node in 90% of cases. Inferior to the crux along the posterior interventricular groove, the RCA gives off a branch known as the posterior descending artery (PDA) in about 85% of patients (Fig. 82-4, B). The PDA supplies the posteroseptum and inferior wall of the right and left ventricles. A dominant RCA by definition supplies the PDA and continues beyond the crux along the left posterior AV groove to terminate into one or several posterolateral branches. In 10% of patients, the PDA is a continuation of the left coronary system (predominantly the LCx, i.e., the left dominant coronary system). In about 5% to 10% of hearts, the RCA and LCx share the same blood supply to the diaphragmatic surface of the heart (i.e., a co-dominant coronary system).5 The definition of coronary segments is important, especially in reporting the results of CT coronary angiography. Sometimes it can be difficult to define a particular segment of the coronary artery tree or the branches of the main coronary segments. Specifying the exact location of the disease and the segment involved as precisely as possible is important in communicating with the treating physicians, especially in coronary arteries with multiple lesions. Several classifications are used to define coronary segments, such as the 26-segment Coronary Artery Surgery Study (CASS) system and 15-segment American Heart Association (AHA) system.6,7 Figures 82-5, A and B illustrate the nomenclature of coronary arteries based on a modified 16-segment AHA classification.8 Coronary arteries with aberrant origins are known as anomalous coronary arteries (ACA). The frequency of ACA varies from 0.3% to 5.6% depending on the definition of ACA in each individual study.9 Studies with more stringent criteria only accept coronary arteries as anomalies if they originate from the opposite coronary sinuses of Valsalva. The less stringent studies may include coronary arteries with ectopic origins at their respective coronary sinuses. The most frequent forms of ACAs in descending order are an RCA originating from either the right or left sinus of Valsalva (Fig. 82-6, A), an LCx arising from the right coronary sinus or RCA (Fig. 82-6, B), and an LCA arising from the right sinus of Valsalva.9 The clinical outcome of ACAs is not always benign. Just like any coronary artery, ACAs may undergo atherosclerosis and become the culprit artery for ischemic heart disease.10 Failure to adequately identify and assess ACAs may lead to misdiagnosis and failure to appropriately treat the culprit artery. More significantly, ACAs are associated with sudden death. It has been reported that 12% to 31% of all cases of sudden death in young athletes/military recruits in the North American population may be associated with ACAs.9,11–14 Most sudden deaths associated with ACA occur during or after physical exercise. Although the exact mechanism of cardiac ischemia in such cases is unclear, it has been postulated that interarterial ACAs with a proximal course between the aorta and pulmonary arterial trunk (so-called malignant coronary anomaly) may be compressed during or after physical exercise (see Fig. 82-6, A). ACAs with an interarterial course can be corrected by surgical reimplantation of coronary arteries to their respective coronary sinuses of Valsalva.15 Therefore, early detection of malignant ACA may lead to prevention of sudden death in adolescents or young athletes with this condition. The cardiac veins, in general, follow the distribution of the coronary artery system, running parallel and superficial to the coronary arteries and their branches. The great cardiac vein runs parallel but in opposite direction to the LAD in the anterior interventricular groove (see Fig. 82-3, A). The great cardiac vein continues its course in the left anterior AV groove along with the LCx artery (see Fig. 82-3, A). Throughout it course, the great cardiac vein receives its tributaries from the left ventricle and atrium. In the left posterior AV groove, the great cardiac vein becomes a larger venous structure known as the coronary sinus (see Fig. 82-3, B). The coronary sinus is about 3 to 5 mm in diameter and 2 to 5cm in length and receives blood from most cardiac veins before it empties into the right atrium. One of its major tributaries is the middle cardiac vein, which runs superiorly in the posterior interventricular groove along the PDA (see Fig. 82-3, B). It empties into the coronary sinus at the crux of the heart. The lateral, posterolateral, and posterior cardiac veins of the left ventricle receive blood from their respective aspects of the left ventricle before emptying into the coronary sinus along the left AV groove. There are multiple anterior cardiac veins that receive blood from the right ventricle and drain into the small cardiac vein. The small cardiac vein runs along the right AV groove downward toward the crux, where it subsequently empties into the coronary sinus. Some of anterior cardiac veins drain directly into the right atrium. Identification of the coronary sinus and its tributaries are important in the electrophysiologic study of the cardiac conduction system. The coronary sinus is a frequent site for placing a pacing catheter or electrode in an electrophysiologic study. In patients with heart failure and dyssynchrony between the right and left ventricles, cardiac resynchronization therapy (CRT) with biventricular pacing can help resynchronize the right and left ventricles and hence improve cardiac output, heart failure symptoms, and survival.16,17 Biventricular pacing involves placement of a pacing electrode into one of the anterolateral tributaries of the great cardiac vein or posterior vein of the left ventricle via the coronary sinus.
Vascular Anatomy of the Thorax, Including the Heart
Systemic Arteries
Ascending Aorta and Its Branches
Radiologic Observations
Coronary Arteries
Clinical Importance
Coronary Artery Nomenclature
Anomalous Coronary Arteries
Clinical Significance of Anomalous Coronary Arteries
Cardiac Veins
Vascular Anatomy of the Thorax, Including the Heart