Stent Grafts
Zenith Endograft
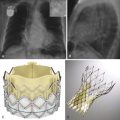
The Zenith endograft (Cook Medical, Inc., Bloomington, Ind) was released in 2003 ( Fig. 19-1 ). This Food and Drug Administration (FDA)–approved woven polyester device, which consists of a stainless steel main body with a diameter of 22 to 36 mm and a length of 11.2 to 17.9 cm, allows suprarenal fixation. It is suitable for an abdominal aortic aneurysm (AAA) with a neck diameter of 18 to 28 mm, a length of 15 mm or more, and a neck angle of less than 60 degrees. The device is delivered through an integrated 20- to 26-Fr sheath. The Zenith endograft is unique in that it offers multiple combinations of lengths and diameters. Although it has a more complex delivery system, its newest design adds more flexibility with less kinking of limbs. This device is MRI conditional ( Box 19-1 ).

- ▪
MRI safe: An item that poses no known hazards in all MRI environments. MRI safe items include nonconducting, nonmagnetic items such as a plastic Petri dish. An item may be determined to be MRI safe by providing a scientifically based rationale rather than test data.
- ▪
MRI conditional: An item that has been demonstrated to pose no known hazards in a specified MRI environment with specified conditions of use. Field conditions that define the specified MRI environment include field strength, spatial gradient, dB/dt, radiofrequency fields, and specific absorption rate. Additional conditions, including specific configurations of the item, may be required.
- ▪
MRI unsafe: An item that is known to pose hazards in all MRI environments. MRI unsafe items include magnetic items such as a pair of ferromagnetic scissors.
dB/dt, The ratio between the amount of change in amplitude of the magnetic field (dB) and the time it takes to make that change (dt); MRI, magnetic resonance imaging.
Talent Endograft
The Talent endograft (Medtronic, Minneapolis, Minn) was FDA approved in 2008 ( Fig. 19-2 ). This polyester graft sewn to a nickel titanium (nitinol) frame is characterized by a main body diameter of 22 to 36 mm and a main body length of 14 to 17 cm. The primary indication is for AAA with a neck length of at least 10 mm, a neck diameter of 18 to 32 mm, and a neck angle of less than 60 degrees. Its flexibility and suprarenal fixation make Talent a good choice for aortic aneurysms that are an anatomic challenge because of a short neck and large diameter. One of the main disadvantages of this endovascular device is its large delivery profile with an integrated sheath of 22 to 24 Fr. This device is MRI conditional (see Box 19-1 ). It can be identified on x-ray film by the uncovered suprarenal fixation spikes.

Endurant Endograft
The Endurant endograft (Medtronic) was FDA approved in 2010 ( Fig. 19-3 ). This polyester graft sewn to a nitinol frame has a main body diameter of 23 to 36 mm and a length of 12.4 to 16.6 cm. This endograft is suitable for an AAA with a neck diameter of 19 to 32 mm, a neck length of at least 10 mm, and a neck angle of less than 60 degrees. This graft has a suprarenal fixation and is delivered through an 18- to 20-Fr delivery sheath. It a good option for AAA with short necks and challenging anatomy. This device is MRI conditional (see Box 19-1 ). It can be identified on x-ray film by the uncovered suprarenal fixation spikes.

AneuRx Stent Graft
The AneuRx device (Medtronic) is an FDA-approved polyester-woven nitinol endograft with an infrarenal fixation and consists of a main body diameter of 20 to 28 cm and a main body length of 13.5 or 16.5 cm. It was approved for an AAA of 18 to 25 mm neck diameter and a neck length of 10 mm or more ( Fig. 19-4 ). This device was released in 1999 for its use, but in 2003, a revision of the instruction for use changed the neck length to greater than 15 mm. This device has a delivery profile that consists of an integrated sheath of 21 Fr. One of the main disadvantages of the AneuRx is its high incidence of migration. This device is MRI conditional (see Box 19-1 ).

Excluder Endograft
The Excluder endograft (Gore Medical, Flagstaff, Ariz) was FDA approved and released in 2002 ( Fig. 19-5 ). Its fabric material consists of expanded polytetrafluoroethylene (ePTFE). The main body has a diameter of 23 to 31 mm and a length of 12 to 18 cm. This device is suitable for an AAA with a neck length of at least 15 mm, a neck diameter of 19 to 26 mm, and a neck angle of less than 60 degrees. In contrast to other stent grafts, the Excluder is delivered through a separate sheath of 18 to 20 Fr and has a limited infrarenal fixation. The main advantage of this graft is its small profile, which makes it easy to deploy. This device is MRI conditional (see Box 19-1 ).

Anaconda Stent Graft
The Anaconda device (Vascutek, Renfrewshire, Scotland) is a trimodular stent graft and consists of a woven Dacron prosthesis supported by self-expanding nitinol ring stents ( Fig. 19-6 ). The main body is available in diameters between 19.5 and 34 mm and lengths between 6 and 6.5 cm. This endograft has an active fixation system consisting of four pairs of hooks. The endograft is delivered through a 20.4 to 22.5 Fr sheath. This device is MRI conditional (see Box 19-1 ).

Endologix Endograft
The Endologix device (Endologix, Irvine, Calif) is an FDA-approved endograft that was released in 2004 ( Fig. 19-7 ). This ePTFE device has a main body diameter of 22 to 28 mm and a length of 10 to 15.5 cm. It is approved for treatment of an AAA with an aortic neck diameter of 18 to 26 mm and a length of at least 15 mm, with an angle of less than 60 degrees. This graft is delivered through an integrated sheath of 21 Fr and has a fixation that sits on the aortic bifurcation with infrarenal or suprarenal extensions. Its unibody design with a unique support at the aortic bifurcation and a one side percutaneous make this device an attractive choice. The main disadvantages are the lack of sizing versatility and the minimal radial force that increases the risk of migration. This device is MRI conditional (see Box 19-1 ).

Vanguard Stent Graft
The Vanguard device (Boston Scientific, Natick, Mass) is a second-generation modular stent graft consisting of a nitinol metal framework covered with polyester graft material ( Fig. 19-8 ). This graft was first implemented in 1994. Vanguard comes with a main body diameter of 22 to 30 mm and iliac components of 10 to 12 mm. It is delivered through an 18-Fr sheath. The contralateral limb is delivered through a 10-Fr sheath by a percutaneous approach. The indication for this device is an infrarenal AAA with a neck of 20 to 25 diameter, a length of at least 15 mm, and neck angulation less than 60 degrees. This device is MRI safe (see Box 19-1 ).

Ancure Endograft
The Ancure device is a unibody nonsupported woven polyester endograft (Guidant Corp., St. Paul, Minn) with a main body diameter of 26 mm ( Fig. 19-9 ). This endograft is suitable for an aneurysm with an aortic neck diameter of 18 to 26 mm and a neck length greater than 15 mm. It is delivered through a 24-Fr introducer sheath. Although it was approved in 1999 by the FDA for the repair of AAA, Guidant suspended production of this device and announced a recall of all existing inventory in 2001. This device is MRI safe (see Box 19-1 ). It is easily identified by the absence of metalwork within the main body.

Talent Thoracic Endograft
The Talent thoracic endograft (Medtronic) is made of thin woven polyester fabric sewn to a self-expanding nitinol wire frame ( Fig. 19-10 ). This device comes in four different configurations: proximal main, proximal extension, distal main, and distal extension. It has numerous device diameters, ranging from 22 to 46 mm, and lengths of 11.2 to 11.6 cm. The device profile requires 20- to 24-Fr introducer sheaths. A minimum aortic neck diameter of 20 mm is required at the sealing zone. This device is MRI conditional (see Box 19-1 ).

Conformable GORE TAG Thoracic Endoprosthesis
The Conformable GORE TAG thoracic endoprosthesis (Gore Medica; Fig. 19-11 ) is made of an expanded ePTFE tube that is reinforced with an ePTFE and fluorinated ethylene propylene (FEP) film and an external nitinol self-expanded stent affixed to the graft. This device is available in a diameter of 26 to 40 mm and a length of 10 to 20 cm. The device profile requires 20- to 24-Fr introducer sheaths. A minimum aortic diameter of 20 mm is required at the sealing zones on either end. This device deploys from the middle of the graft toward each end, a design characteristic intended to prevent wind-socking at the proximal fixation zone. This device is MRI conditional (see Box 19-1 ).

Zenith Thoracic Stent Graft
The Zenith thoracic endograft (Cook Medical, Inc.) has a stainless steel body frame and a polyester covering ( Fig. 19-12 ). It has proximal and distal components and extension elements of varying sizes. It is MRI conditional (see Box 19-1 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree