CHAPTER 111 Vascular Imaging of Hepatic Transplantation
With more than 6000 surgeries performed in 2008,1 liver transplantation is the current treatment of choice for many causes of progressive acute and chronic end-stage liver disease. In the United States, liver transplantation typically involves harvesting of an organ from a deceased donor (orthotopic liver transplantation). However, demand for liver transplantation is increasingly outstripping the supply of cadaveric livers, and transplantation of a portion of the liver from a live donor (i.e., living related donor) is increasing in popularity. Knowledge of recipient and live donor hepatic vascular anatomy is critical for proper preoperative planning for transplantation.
Although malignant disease is a contraindication to transplantation in most other solid organs, liver transplantation offers the only potential cure for primary, low- and moderate-volume hepatocellular carcinoma. The overall 5-year survival in this setting is expected to be 70%, with a recurrence rate below 15%. Compared with hepatocellular carcinoma, transplantation for cholangiocarcinoma is controversial because 5-year survival rates are much lower (20% to 30%) and recurrence rates are much higher. Transplantation for metastatic liver lesions is generally not performed; rare exception is made for metastatic carcinoid and other neuroendocrine tumors.2
PRETRANSPLANTATION WORK-UP
Imaging of Liver Transplant Recipients
The radiologic evaluation of a potential candidate for orthotopic liver transplantation primarily focuses on an assessment of the degree of cirrhosis (liver size and morphology, presence of ascites) and patency and caliber of the portal vein and associated feeding veins (splenic vein and superior mesenteric vein) to determine whether a suitable site for venovenous anastomosis between the donor liver and recipient portal vein or feeding branches exists. Because of the greatly increased risk for development of hepatocellular carcinoma in cirrhotic livers, most centers perform routine sonographic surveillance for early detection of hepatocellular carcinoma. Whereas ultrasonography is sensitive in the evaluation of portal vein caliber, patency, and direction of flow, its operator-dependent nature limits its sensitivity for detection of hepatocellular carcinoma (20% to 40% for lesions smaller than 3 cm).3
Dynamic contrast-enhanced (multiphase) multidetector computed tomography (CT) and magnetic resonance imaging (MRI) enable rapid, whole-liver evaluation during arterial and portal venous phases of intravenous contrast enhancement, allowing a comprehensive assessment of the hepatic artery and the portal, splenic, and superior mesenteric veins as well as major collateral veins. In general, both contrast-enhanced CT and MRI are more sensitive than sonography for detection of hepatocellular carcinoma lesions larger than 2 cm (sensitivity, 74% to 96%). The 2-cm size is important because by the Model for End-Stage Liver Disease scoring system, cirrhotic patients with 2-cm or larger lesions are assigned priority for liver transplantation. Although both multiphase CT and MRI are less sensitive for detection of hepatocellular carcinoma lesions smaller than 2 cm, they are better than ultrasonography, which has sensitivities as low as 20% for these lesions.4–9 MRI benefits from its ability to provide additional lesion characterization for further differentiation of the myriad types of liver nodules in cirrhotic livers based on signal intensity (e.g., T1 and T2) and contrast enhancement characteristics. Multiphase CT and MR examinations can determine vascular patency and are superior to ultrasound examination in the characterization of the recipient vascular anatomy.
Imaging of Potential Living Donors for Hepatic Transplantation
As an alternative to cadaveric liver transplantation, which is not an option in many European and Asian countries and usually entails a long wait time in many regions of the United States, living related and living donor hepatic transplantations have been advocated to expand the pool of available hepatic allografts. In adult-to-adult living donor transplantation, the right lobe (segments V, VI, VII, and VIII) is resected and transplanted. In adult-to-pediatric liver donation, the left lateral segment (segments II and III) is transplanted into the pediatric recipient. However, because of concerns of donor safety in the United States, fewer than 250 adult living donor hepatic transplantations were performed in 2007.10
Approximately 55% of patients have a conventional right and left hepatic artery arising from a common hepatic artery (Michel type I, Fig. 111-1).11,12 However, in the remaining 45% of patients, there can be a wide range of hepatic artery variations (Fig. 111-2) that can be described by the Michel classification (Table 111-1). Proper identification of hepatic artery anatomy in the donor is critical for surgical planning and minimization of postoperative complications in both the donor and recipient. A donor may be rejected when aberrant arterial anatomy is also associated with other biliary and venous variants, which in combination result in an increased complexity of the operation.
Table 111-1 Michel Classification of Arterial Variants
| Type 1 Normal anatomy: proper hepatic artery dividing into sole right and left hepatic arteries |
| Type 2 Left hepatic artery replaced to the left gastric artery |
| Type 3 Right hepatic artery replaced to the superior mesenteric artery |
| Type 4 Both right and left hepatic arteries replaced |
| Type 5 Accessory left hepatic artery |
| Type 6 Accessory right hepatic artery |
| Type 7 Accessory right and left hepatic arteries |
| Type 8 Proper hepatic artery originating from the superior mesenteric artery combined with an accessory left hepatic artery |
| Type 9 Proper hepatic artery arising from the superior mesenteric artery |
| Type 10 Proper hepatic artery arising from the left gastric artery |
From Artioli D, Tagliabue M, Aseni P, et al: Detection of biliary and vascular anatomy in living liver doners: value of gadobenate dimeglumine enhanced MR and MDCT angiography. Eur J Radiol. In press.
Ideal portal venous anatomy has the right portal vein branching from the main portal vein (Fig. 111-3) and not from the left portal vein. Cases in which portal venous branches to segment IV arise from the right anterior portal vein may be a contraindication to transplantation.
Ideal hepatic venous anatomy has the single dominant right hepatic vein available for anastomosis without significant (>5 mm diameter branch veins) hepatic venous branches draining into the now-missing middle hepatic vein. However, many variants in hepatic venous drainage exist. Hepatic venous branches larger than 5 mm that drain the right posterior lobe (VII and VI) and insert into the inferior vena cava (IVC) may require separate venous anastomoses in the recipient to prevent hepatic segmental congestion or bleeding and subsequent liver dysfunction. Hepatic venous branches larger than 5 mm that drain the right anterior lobe branches (VIII and V) into the middle hepatic vein will invariably transect the surgical resection plane and probably require separate venous anastomoses to also prevent complications such as hepatic venous congestion in the recipient.13,14
SURGICAL ANATOMY (CADAVERIC LIVER TRANSPLANTATION)
The hepatic artery anastomosis is typically a branch patch anastomosis formed at the origin of the gastroduodenal artery from the common hepatic artery in the recipient and at the origin of the splenic artery from the celiac trunk in the donor (Fig. 111-4). In some instances, a donor anastomosis is made at the celiac axis with use of an aortic patch (Carrel patch).15,16 On occasion, an aortic jump graft will be formed from the donor common and external iliac arteries joined to the recipient abdominal aorta in an end-to-side anastomosis and to the hepatic artery of the donor by a branch patch anastomosis. This technique can be used in cases of small native hepatic artery or celiac artery stenosis in which an adequate inflow cannot be ensured.
When there is variant donor hepatic artery anatomy, modification of normal technique is required. For example, in Michel type III variant (a replaced right hepatic artery from the superior mesenteric artery, the most common arterial variant; see Fig. 111-2),17 a primary anastomosis between the donor celiac artery with an aortic patch and the recipient branch patch at the gastroduodenal artery takeoff and a secondary anastomosis between the replaced right hepatic artery and the proximal stump of the donor splenic artery can be performed.15 In situations of variant hepatic arterial anatomy in the recipient, such as a replaced right hepatic artery, the larger of the two inflow vessels is used.16
The portal vein anastomosis is typically an end-to-end anastomosis between the donor and recipient portal veins.18 A venous jump graft between the donor portal vein to the recipient superior mesenteric vein may be needed in cases of portal vein thrombosis or prior portal venous surgery.16 Rarely, arterialization of the portal vein, in which the donor portal vein is anastomosed to the arterial vessels of the recipient, has been performed when a suitable visceral venous anastomosis is impossible because of extensive thrombus.19
For the caval anastomosis, the IVC is transected above and below the intrahepatic segment during cadaveric hepatectomy, and an end-to-end anastomosis is made between the upper and lower margins of the IVC in the recipient.16 In the “piggyback” technique, a single end-to-end anastomosis is made between the donor hepatic vein–IVC stump and the recipient’s common hepatic vein stump off the IVC (Fig. 111-5).18 This technique is preferred at some institutions because IVC flow is not interrupted during most of the operation, thus reducing inherent operative risk as well as obviating the need for venovenous bypass.20
COMPLICATIONS AFTER LIVER TRANSPLANTATION
Orthotopic liver transplantation is a technically complex procedure with inferior vena caval, portal venous, and hepatic arterial vascular anastomoses. A variety of short- and long-term complications may arise. Of particular relevance to interpreting physicians are the vascular complications, which are among the most frequent causes of early acute graft failure.21 The transplant team is particularly sensitive to the patency of the allograft vessels in the immediate postoperative period in patients with signs of bile leak or sepsis or more commonly a persistent elevation in serum liver enzymes. When any of these are suspected, ultrasonography with color, power, and spectral Doppler imaging is immediately performed. If sonography is inconclusive or suggests arterial stenosis or occlusion, MR angiography (MRA) or CT angiography (CTA) is typically performed to determine the extent of these potentially morbid vascular complications to enable revascularization with endovascular interventional procedures.
Both CTA and MRA are performed for definitive evaluation of vascular complications after liver transplantation, with a sensitivity reported to be 100%, specificity between 74% and 96%, and negative predictive value of 100%.22–27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


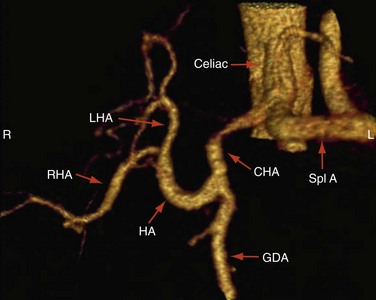
 FIGURE 111-1
FIGURE 111-1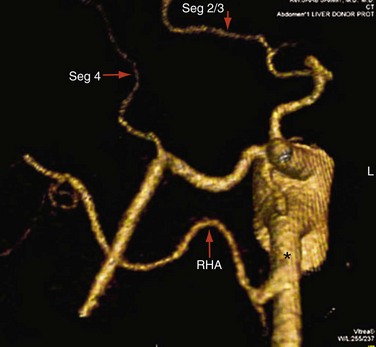
 FIGURE 111-2
FIGURE 111-2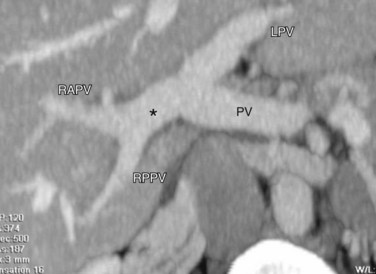
 FIGURE 111-3
FIGURE 111-3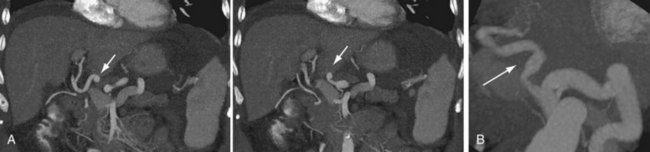
 FIGURE 111-4
FIGURE 111-4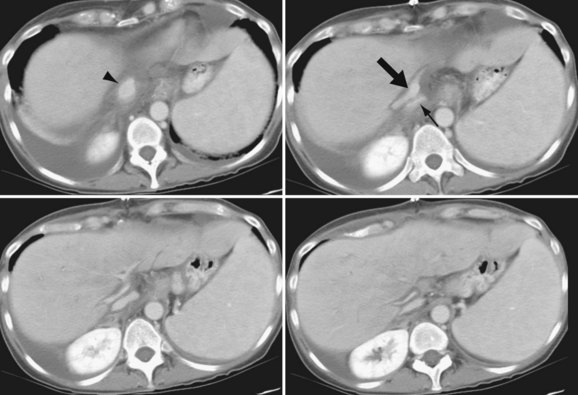
 FIGURE 111-5
FIGURE 111-5