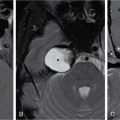
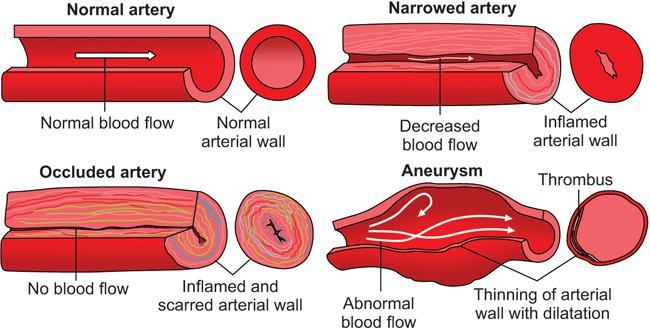
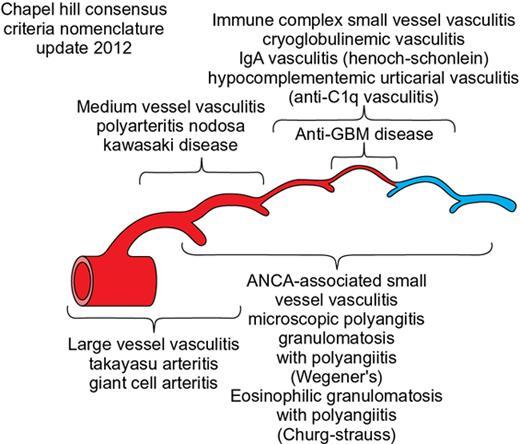
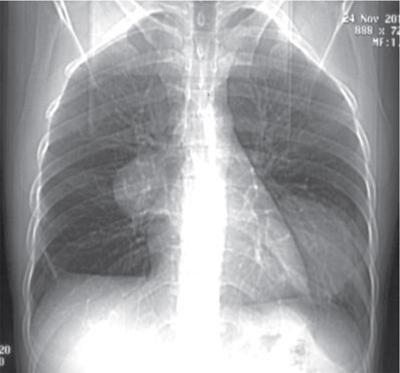
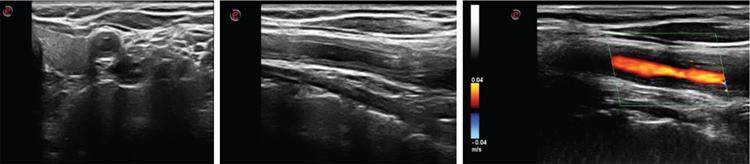
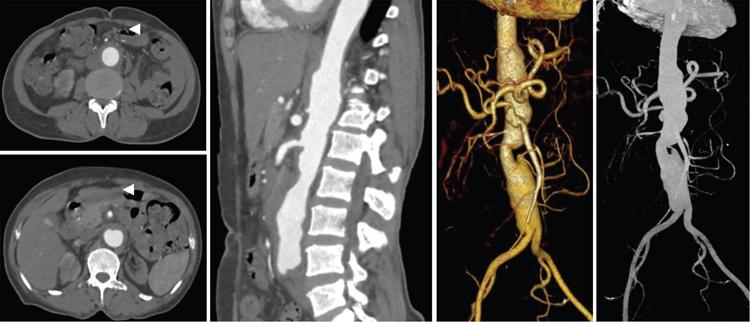
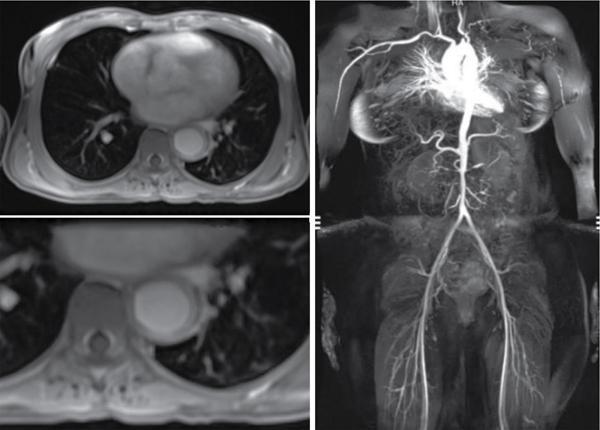
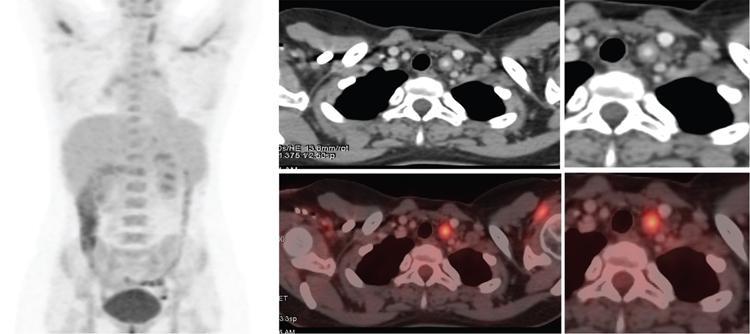
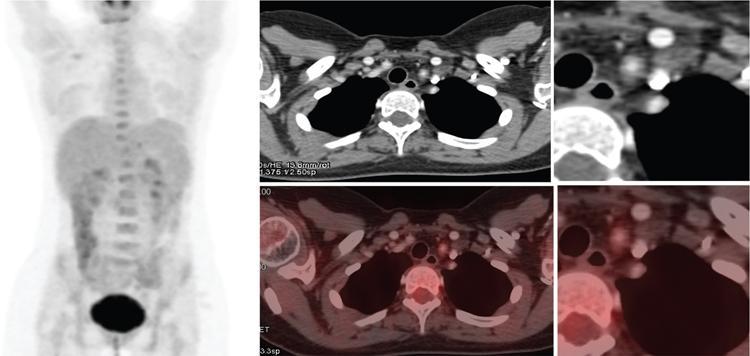
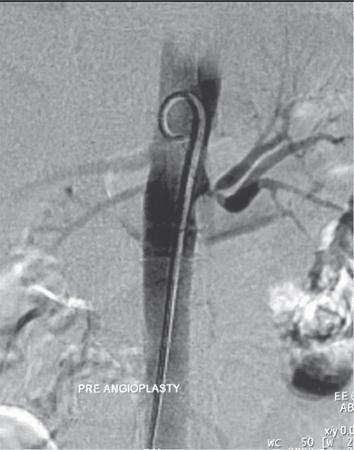
S. Rammurti, S. Gayatri, P. Krishna Mohan, K. Sreshta, N. Kavitha Vasculitides include an uncommon group of diseases characterized by relapsing and remitting immune-mediated inflammation of vessel walls. The vasculitides can be divided into primary and secondary vasculitides, depending on the etiology and according to the size of the vessel affected. Primary vasculitis is of unknown origin. Secondary vasculitis could be related to infection, malignancy, connective tissue disease, exposure to drugs or environmental exposure. Inflammation of vessel wall leads to vessel wall damage and weakening resulting in the formation of aneurysm or stenosis and occlusion of the lumen leading to tissue infarction. Vasculitis affects patients of all ages and poses unique diagnostic and therapeutic challenges. Management comprises establishing the diagnosis of true vasculitis ruling out vasculitis mimics, defining the etiology if feasible, assessing the status of vessels involved including the stage of inflammation, deciphering the pathological process of vessel damage, investigating for the presence and extent of systemic involvement and finally planning therapy in the background of co-morbidities (Fig. 2.3.1). The diagnosis of vasculitis requires a high index of suspicion, especially in the systemically unwell patient with multiorgan involvement. Symptoms and signs of vasculitis depend upon the vessels involved. Large vessel vasculitis typically presents with limb claudication, absent pulses and unequal blood pressure, while small vessel vasculitis presents with palpable purpura and proteinuria. Medium vessel vasculitis which involves main visceral arteries and their initial branches produce symptoms according to the involved vessels and the circulation. In LVV the inflammatory response begins at the adventitia and spreads inward toward medial and intimal layers. This results in the development of occlusive and aneurysmal vascular lesions. In contrast, small vessel inflammation typically begins with endothelial injury and intimal inflammation, which propagates outward to the media and adventitia. Further spread to the adventitia results in panarteritis, although this is more typical of MVV, which shares features of both. While SVV is characterized by the early inflammatory injury of the intima followed by activation of the coagulation cascade in small caliber vessels resulting in the thrombotic and necrotizing pathology. As the treatment using steroids and cytotoxic agents is potentially toxic, establishing a proper diagnosis is important before instituting the therapy. Tissue biopsy is often not feasible due to limitations of access, except from the temporal arteries. Emergence of imaging modalities with the potential to monitor inflammation of the vessel is helpful for evaluating the extent and activity of the disease. Invasive imaging by catheter angiogram helps in guiding the surgical or endovascular management. The relapsing and remitting course of the disease with structural alteration in the vessel also poses a challenge to the management by surgical or endovascular intervention. Successful management also involves regular monitoring to foresee complications arising from the disease process itself, the medical management or the intervention. International Chapel Hill Consensus Conference (CHCC) put forth a nomenclature system which specifies the findings that have to be observed for classifying a patient into a particular category of vasculitis. Names and definitions for common forms of vasculitis were first proposed by the CHCC in 1994 which have been revised in 2012. The CHCC 1994 categorized vasculitis based on the size of affected blood vessels into large, medium and small vessel vasculitis. The CHCC 2012 added new categories of variable vessel vasculitis, single organ vasculitis, vasculitis associated with systemic disease and vasculitis associated with probable etiology to the nomenclature system (Table 2.3.1). Moreover, in the recent nomenclature, eponyms were preferentially replaced with descriptive terms that had pathophysiological specificity. Modified from J. C. Jennette, Overview of the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides, Clin. Exp. Nephrol. 17 (2013) 603–606. The CHCC nomenclature divides vasculitis depending on a combination of various features including the etiology, clinical features, demographics, pathogenesis, type of affected vessel, type of inflammation, organ distribution and genetic predisposition. Based on the etiology, vasculitis can be broadly divided into infectious and non-infectious types. CHCC only deals with vasculitis which is non-infectious, i.e. vasculitis which is not caused by direct pathogenic invasion of the vessel wall. Imaging is an important tool in the diagnosis and monitoring of vasculitis. However, imaging findings are not specific and correlation with clinical and laboratory findings is important in the final diagnosis. Traditionally, angiography is the gold standard imaging modality for diagnosis of vasculitis. It is now increasingly being replaced by cross-sectional imaging studies. The choice between various imaging studies depends on many factors like concern for radiation exposure (especially in children), patient tolerance to specific contrast agents, need for intervention, requirement of follow-up imaging and type of vasculitis suspected clinically (Fig. 2.3.2). Non-specific findings seen on chest radiography in vasculitis include cardiomegaly, nodules, infiltrates, consolidation and pleural effusions. In Takayasu arteritis where the aorta is involved, radiography may show irregular contour of the descending aorta, aortic wall calcification, dilated aortic arch, calcification of wall of left subclavian artery and rib notching (Fig. 2.3.3). Grey-scale sonography has the highest resolution among all the non-invasive imaging techniques used in the assessment of vasculitis to depict changes in the vessel wall. Doppler sonography is used to evaluate vascular hemodynamics including the quantitative evaluation of the flow direction and velocity. Qualitative evaluation of flow waveforms is useful for indirect assessment of distal or proximal pathology. Ultrasound is most useful in evaluation of superficial vessels, including arteries of the upper and lower limbs, great vessels of the arch of aorta, temporal artery and the circle of Willis through transcranial doppler. Ultrasound is a valuable imaging tool in large-vessel vasculitis. It depicts inflammatory wall thickening and helps in establishing early diagnosis which may prevent complications. Temporal arteries can be evaluated through ultrasound, which shows hypoechoic edematous wall thickening (“halo sign”) in acute temporal arteritis. Ultrasound is also useful in assessment of axillary arterial involvement which helps in the diagnosis of large-vessel giant cell arteritis. The carotid vessels, vertebral arteries, subclavian arteries and the abdominal aorta can also be evaluated in cases of Takayasu arteritis and other types of aortitis. Medium vessel vasculitis frequently presents with visceral artery aneurysms which may be detected on ultrasound when involving the proximal arteries. Lesions of coronary arteries common in children with Kawasaki syndrome can be detected on echocardiography (Fig. 2.3.4). In small vessel vasculitis ultrasound is important in determining the disease extent and activity. Cardiac, pleural and renal involvement can be evaluated. Ultrasound however has the disadvantage of high operator dependence and a limited field of view. Assessment of the thoracic aorta that is commonly involved in giant cell arteritis and Takayasu arteritis is limited. Endoluminal ultrasound can evaluate the heart and thoracic aorta using transesophageal probes and can evaluate the large and medium vessels from within the lumen using endovascular probes. Contrast-enhanced ultrasound is useful in the diagnosis and monitoring of vasculitis as it can provide information about inflammation of the vessel wall. It is a useful tool to assess vessel wall vascularization. CT and CT angiography (CTA) can detect pathological changes in large, deep vessels with a convenient scanning time and good spatial resolution. CT can assess the vessel wall and the lumen, and it can therefore show changes in the vessel wall which are not seen on conventional angiography. Three-dimensional reconstruction can produce angiogram-like images, depicting areas of occlusion, stenosis and aneurysmal dilatation (Fig. 2.3.5). CT can measure accurate diameter of vessels, detect mural calcifications and concentric mural thickening, a classic finding in large vessel vasculitis. Arterial wall enhancement on CTA has been linked to disease activity in a few studies, however other studies with differing results have been reported. Role of CT in monitoring disease course has also been proposed, according to which there is a resolution in wall enhancement after successful treatment. CT is also useful in broad evaluation of the other organs and the retroperitoneum for related pathologies. Cardiac CT is useful in accurate evaluation of the heart and coronary arteries which are commonly involved in Kawasaki disease. CT pulmonary angiography is the imaging technique of choice to evaluate pulmonary arterial involvement and its associated complications like thrombosis and aneurysms. High resolution CT (HRCT) of chest helps in the documentation of lung parenchymal changes which are frequently present in small vessels vasculitis. HRCT of the paranasal sinuses is of diagnostic value in patients of Granulomatosis with polyangiitis (GPA) with sino-nasal involvement. CT is useful for demonstrating end-organ changes and complications of vasculitis. Abnormalities may be noted in the brain, heart, lungs, liver, kidneys or bowel, depending on the specific type of vasculitis. Dual energy CT with reconstruction of virtual monoenergetic images and material decomposition images can help reduce dose of contrast agent required and can improve vessel enhancement. Vessel wall calcification can be subtracted by acquisition of iodine/calcium material decomposition images, with improved delineation of the vessel lumen. Iodine/water material decomposition images can be used for assessment of organ perfusion. Evaluation of vasculitis by MRI may include plain and contrast-enhanced MR, MR angiography, vessel wall imaging and in some cases myocardial imaging. MRI provides multiplanar imaging and 3D reconstruction capability without risk of exposure to ionizing radiation and without the use of iodinated contrast. Although spatial resolution of MR is inferior to CT, it provides superior soft tissue contrast. In addition to identifying increased vessel wall thickness (Fig. 2.3.6), MR can detect vessel wall oedema on T2 sequences, and it can detect fibrosis on post-contrast studies. MR angiography is used for focussed vascular imaging to produce images of the vessel lumen like conventional angiography. Vascular imaging in MRA is possible with non-invasive techniques such as phase-contrast or time of flight techniques. However, minimally invasive contrast MRA provides better arterial detail. MRA images of vessel lumen must be obtained at a precise timing after contrast injection. Delayed post-contrast images are obtained for evaluation of vessel wall thickening and wall enhancement, which correlates with active inflammation. Similar to CT, MR can be used to evaluate large, deep vessels like the aorta and its branches. Degree of arterial wall thickening and vessel wall oedema can be assessed which have been linked to disease activity. MR can assess wider vasculature for defining the disease extent and is useful for longitudinal monitoring of the vessel structure. High resolution MRI has comparable sensitivity and specificity to HRUS in detecting temporal artery involvement in giant cell arteritis. Lack of ionizing radiation and non-invasive nature make MR preferable in children with Kawasaki disease. Phase-contrast and cine MR are the gold standard in evaluation of myocardial function and valvular regurgitation. MR can depict granulomatous vasculitis in the paranasal sinuses, nasal cavity, mastoid and orbits. It is highly sensitive in detection of pathologies in primary CNS vasculitis (PCNSV), however it has a low specificity as the findings are largely non-specific. Diffusion weighted imaging (DWI) can detect restriction of water molecule movement in biologic tissues. Increased cellular infiltrate in vasculitis may cause diffusion restriction within the vessel wall. DWI in combination with PET scan can differentiate between active and inactive disease. Vessel wall imaging can depict inflammatory changes in the intracranial vessels in CNS vasculitis. The affected vessels show smooth, concentric wall enhancement. Vessel wall imaging can also help differentiate intracranial atherosclerotic disease from vasculitis. The affected vessels show smooth, concentric wall enhancement. Vessel wall imaging can also help differentiate intracranial atherosclerotic disease from vasculitis. Eccentric wall thickening and enhancement favour atherosclerotic disease as opposed to concentric enhancement seen in vasculitis. MR angiography is used for focussed vascular imaging to produce images of the vessel lumen like conventional angiography. Vascular imaging in MRA is possible with non-invasive techniques such as phase-contrast or time of flight techniques. However, minimally invasive contrast MRA provides better arterial detail. MRA images of vessel lumen must be obtained at a precise timing after contrast injection. Delayed post-contrast images are obtained for evaluation of vessel wall thickening and wall enhancement, which correlates with active inflammation. The cross-sectional functional imaging with PET (Positron Emitting Tomography) using 18F labelled glucose analogue, the Fluro-Deoxy-Glucose (FDG) emerged as an important modality in the diagnostic algorithm of vasculitis. FDG is a positron emitting metabolic contrast which is used extensively in oncology as there is increased accumulation due to increased GLUT receptor expression in cancer cells. However, the inflammatory cells, macrophages also show increased glucose uptake due to increased metabolic rates. This feature has been utilized in inflammation or infection imaging especially in evaluating patients with undiagnosed fever. Vasculitis being one of the four major causes of undiagnosed fever (UDF) or pyrexia of unknown origin (PUO), FDG PET-CT has an established role in diagnosing unsuspected cases of vasculitis. Thus, it occasionally provides early diagnosis of LVV in patients presenting with non-specific symptoms and inconclusive lab investigations. 18F-FDG-PET-CT utility in types of vasculitis other than LVV is not appealing. There is very minimal evidence that it may be useful in detecting small-vessel vasculitis. Multinational Diagnostic and Classification Criteria for Vasculitis (DCVAS) is formed to validate the diagnostic criteria for primary systemic vasculitis. Interpretation of scans depends on the demonstration of increased FDG uptake in the vessel walls of major and minor arteries of thorax, abdomen and extremities. FDG uptake would be continuous in majority of cases of vasculitis. Interrupted uptake in vasculitis is a rare phenomenon unlike in atherosclerotic vessels where the uptake is interrupted and predominantly in lower aorta and lower extremities. This false-positive uptake is a major pitfall in FDG-PET which decreases the specificity. However, correlation with age, CT findings and clinical parameters would help in differentiating the two entities. Degree or grade of FDG uptake is based on the intensity of FDG concentration in relation to liver (Table 2.3.2). Total Vascular Score (TVS) is the sum of uptake in various vascular regions at seven different locations like thoracic aorta, abdominal aorta, subclavian arteries, axillary arteries, carotid arteries, iliac arteries and femoral arteries. Glucocorticoids are the first-line main stay of treatment in all vasculitides except those of infectious etiology. This is a major challenge for FDG PET-CT as the glucocorticoids interfere in FDG uptake, which is found to be decreased with steroid intake as early as within 3 days of starting the therapy. Discontinuation of the steroids at least for a week before would help in eliminating errors while reporting. Monitoring the treatment response while the patient is on medication and during follow-up is an important indication for PET-CT which would show disappearance of abnormal vessel wall uptake. PET-CT being a whole-body study, the extent and distribution of disease is very well documented. FDG-PET would display the inflammatory activity in the vessel wall as increased FDG uptake with high sensitivity and performing CTA would provide high resolution anatomical details of vessel wall which would improve the specificity. CTA provides information about the potential structural complications of vasculitis like aneurysms, dissection, stenosis and their sequalae like cerebral or mesenteric ischaemia, etc. CTA also helps to differentiate vasculitis and atherosclerosis as both demonstrate increased FDG wall uptake. The combined FDG-PET/CTA is a powerful tool in giving both early diagnosis of vasculitis, its activity and potential complications in one stop. However, usefulness of PET-CT in primary diagnosis is still debatable, the functional modality also has an established role in monitoring the treatment response. Hybrid imaging systems PET-CT and PET-MRI further provide additional anatomical information and have proven to be indispensable tools amidst the preferred USG and MR in certain equivocal clinical situations. PET-MRI, a value addition in hybrid imaging, gives functional and anatomical details of the disease process in similar way as PET-CT. Its role is irreplaceable in patients with contraindications to the contrast agents and in paediatric age group with significant low radiation burden. LVV in childhood is a devastating disease predominantly affecting ocular vessels, aorta and its major branches. Delayed diagnosis is due to non-specific symptoms. FDG-PET plays an important role in early diagnosis and determining the extent of disease. PET-MRI has a role in this context in showing the inflammatory component in the vasculature much earlier than complications like occlusions and is also preferred as it decreases radiation to the child. However, availability and cost of the equipment are the limiting factors (Fig. 2.3.7). Nuclear medicine investigations including conventional and hybrid FDG PET predominantly being functional with the radioisotope labelled targeted agents contribute in understanding pathophysiological research studies of leukocyte kinetics and evaluation of therapies, which helped in evolution of newer therapies, particularly immunotherapy. Digital Subtraction Angiography is traditionally the imaging modality of choice for diagnosis of vasculitic disorders. It depicts luminal changes in the vessel clearly with high resolution. Common findings seen on angiography are long, smooth arterial stenosis, occlusion and aneurysms. The extent of disease involvement can be determined by pan-angiography. DSA is the gold standard in diagnosis of polyarteritis nodosa in which multiple aneurysms, segments of ectasia and/or occlusive lesions may be seen. Presence of aneurysms may have a link with the phase or severity of the disease (Fig. 2.3.9). However, early vasculitic changes like thickening of the arterial wall and mural enhancement cannot be demonstrated by DSA and it is therefore not useful in early diagnosis. DSA also has the disadvantages of invasiveness, exposure to ionizing radiation and use of iodinated contrast media. In PCNSV angiographic findings indicating vasculitis include alternating areas of smooth vessel narrowing and dilatation affecting multiple cerebral arteries after ruling out atherosclerosis and other known abnormalities. Diseases with predominant involvement of the small vessels, like IgA vasculitis, may be missed on conventional angiography as it does not have adequate resolution for very small vessels. Arterial injury has a direct effect on prognosis in large vessel vasculitis and the therapeutic goal in vasculitis is to prevent progression of arterial disease. Scoring allows quantitative grading of arterial involvement in large vessel vasculitis. The scores are applicable to CTA and MRA studies. They enable cross-sectional analysis of the type and extent of arterial involvement and monitoring of disease activity over time. According to the proposed scoring system a set of 17 individual arteries are evaluated. Based on luminal data, stenosis and dilatation, scores are generated and then a composite score is calculated by combing the two. Stenosis, dilatation and composite scores quantify arterial involvement and provide an overview of severity and pattern of arterial remodelling. Improvement or worsening of stenosis or dilatation and arterial disease can be analyzed by demonstrating longitudinal change in each score separately. Changes in scores after initiation of treatment can indicate its effectiveness. Moreover, scores can provide an understanding of disease phenotype. According to some studies patients with Takayasu arteritis have higher stenotic and composite scores but lower dilatation scores compared to patients having LV-GCA (large vessel-giant cell arteritis). Scores can also distinguish between early and late large vessel vasculitis (Fig. 2.3.10, Tables 2.3.3 and 2.3.4).
2.3: Vasculitis
Classification of vasculitis
Type of Vasculitis
Disease Entities
Large vessel vasculitis
Takayasu arteritis
Giant cell arteritis
Medium vessel vasculitis
Polyarteritis nodosa
Kawasaki disease
Small vessel vasculitis
IgA Vasculitis
Microscopic polyangitis
Granulomatosis with polyangitis
Eosinophilic granulomatosis with polyangiitis
Variable vessel vasculitis
Behçet disease
Cogan syndrome
Single organ vasculitis
PACNS
Vasculitis associated with systemic disease
SLE
Sjogren syndrome
Rheumatoid arthritis
APLA syndrome
Scleroderma
Vasculitis associated with probable etiology
Infection-induced vasculitis
Acute septic meningitis
Mycobacterium tuberculosis
Neurosyphilis
Viral (HIV-related vasculitis, varicella-zoster vasculopathy)
Fungal (mucormycosis, aspergillosis)
Parasitic (cysticercosis)
Malignancy-induced vasculitis
Drug-induced vasculitis
Radiation-induced vasculitis
Imaging evaluation of vasculitis
Chest radiograph
Ultrasonography
Computed tomography
Magnetic resonance imaging
Nuclear medicine
INTENSITY OF FDG CONCENTRATION
TOTAL VASCULAR SCORE (TVS)
Degree of FDG Uptake
Grade
Degree of FDG Uptake
Score
No uptake
0
Negative
0
Uptake < than mediastinum
1
Minimal but < mediastinum FDG uptake
1
Uptake equal to liver
2
Increased FDG uptake
2
Uptake more than liver
3
Very intense FDG uptake
3
Grade 2 uptake suggests the possibility and Grade 3 is considered positive for the diagnosis of active vasculitis. This is also important in monitoring treatment response.
TVS is the sum of uptake in various vascular regions at seven different locations. The range of TVS would be 0–21 (if uptake in all and locations).
Digital subtraction angiography
Angiographic scores in evaluation of large vessel vasculitis
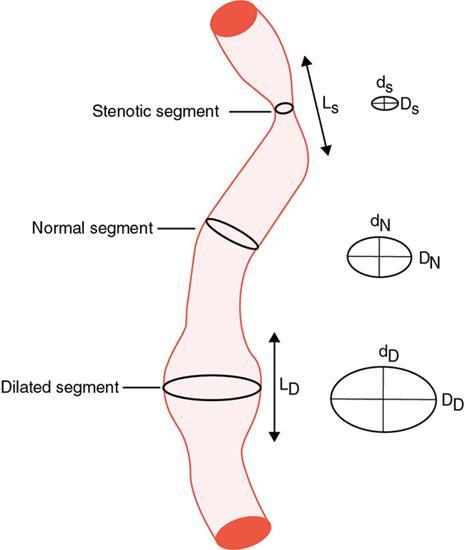
ARTERIES EVALUATED
Ascending aorta
Bilateral axillary arteries
Arch of aorta
Bilateral common carotid arteries
Descending thoracic aorta
Coeliac artery
Abdominal aorta
Superior mesenteric artery
Brachiocephalic artery
Bilateral renal arteries
Bilateral subclavian arteries
Bilateral common iliac arteries
PERCENTAGE OF STENOSIS = 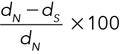
PERCENTAGE OF DILATATION = 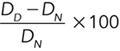
STENOSIS SCORE
Percentage of Stenosis
Points
Length of Artery Involved
Extra Points
<50%
1
<1 cm
0
50–70%
2
1–3 cms
1
≥70%
3
3–10 cms
2
Occlusion
4
≥10 cms
3
DILATATION SCORE
Percentage of Dilatation
Points
Length of Artery Involved
Extra Points
<50%
1
<1 cm
0
50–100%
2
1–3 cms
1
100% or needing procedure
3
3–10 cms
2
≥10 cms
3
ARTERIAL STENOSIS SCORE = Sum of stenosis scores of all the above arteries
ARTERIAL DILATATION SCORE = Sum of dilatation scores of all the above arteries
COMPOSITE SCORE = STENOSIS SCORE + DILATATION SCORE ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vasculitis
Classification of Vasculitis According to the 2012 Revised International Chapel Hill Consensus Conference on the Nomenclature of Systemic Vasculitides
List of the 17 Evaluated Arteries and the Scoring System and Formulas to Calculate Percentage of Stenosis, Percentage of Dilatation, Stenosis Score, Dilatation Score and the Composite Score