The purpose of this article is to review the current state of the art of using vertebral augmentation techniques for treating symptomatic spinal fractures that are associated with malignant lesions and to present potential future trends in treatments for this patient population. Epidemiology and biomechanical ramifications of these lesions are summarized, and treatment regimes, clinical outcomes, complications, and technical issues associated with treatments are presented. Potential future trends and new technologies for performing vertebral body augmentation in patients with metastatic spinal lesions are also discussed in this article.
Metastatic bone tumors in the spine are painful and debilitating but are challenging to treat. Percutaneous approaches for performing vertebral body augmentation for treating metastatic spinal lesions have been developed as good alternatives to open surgery and for pain control. These types of procedures have evolved rapidly during the last 10 years, and the current state of the art has been found to fit neatly into the conventional oncologic treatment algorithm.
Epidemiology and clinical presentation
Spinal metastasis is the most commonly encountered tumor of the spine, occurring in up to 40% of patients with cancer. Each year, 5% of patients with cancer, or approximately 61,000 persons, develop spinal metastasis. The cancers most often metastasizing to the spine include breast (21%), lung (14%), prostate (8%), renal (5%), gastrointestinal (5%), and thyroid (3%) cancers. The posterior half of the vertebral body is usually infiltrated first, with the anterior body, lamina, and pedicles becoming involved later. The treatment regimen for spinal metastasis is generally palliative and consists of a combination of medical therapy (steroids, pain medication, and chemotherapy), radiation therapy, and surgery.
When managing patients affected by spinal metastasis, the primary goals of treatment are to relieve pain and preserve or restore function. Pain is generally described in at least 1 of 3 ways:
- 1.
Constant and localized
- 2.
Radicular
- 3.
Axial, coinciding with functional disability.
Localized pain is generally thought to be a result of periosteal stretch occurring with tumor expansion and is usually treated using radiation because this therapy is effective for decreasing the tumor size. Radicular pain, most likely caused by the tumor pressing against the nerve root, is also addressed using radiation therapy but can be treated using nerve root blocks as well. Axial pain, most frequently associated with mechanical instability of the spine or pathologic vertebral body fracture, is worsened with physical activity but relieved with rest. Axial pain has been traditionally treated by surgically stabilizing the spine.
Biomechanics of pathologic fractures
In patients with spinal metastasis, pathologic fracture can occur under normal physiologic stress. Partial or total destruction of the anterior vertebral body results in decreased load-bearing capacity of the spine. How and when pathologic fracture occurs is generally determined by the size and location of the tumor, the extent of tumor destruction, and the patient’s bone mineral density. Ventrally situated tumors (anterior column) have a potentially greater destabilizing effect than dorsally located masses (middle column) in the presence of intact dorsal elements.
Biomechanics of pathologic fractures
In patients with spinal metastasis, pathologic fracture can occur under normal physiologic stress. Partial or total destruction of the anterior vertebral body results in decreased load-bearing capacity of the spine. How and when pathologic fracture occurs is generally determined by the size and location of the tumor, the extent of tumor destruction, and the patient’s bone mineral density. Ventrally situated tumors (anterior column) have a potentially greater destabilizing effect than dorsally located masses (middle column) in the presence of intact dorsal elements.
The conventional treatment regimen
Radiation Therapy
Radiotherapy has a long history of proven success for alleviating pain in patients with metastatic spinal lesions when they are radiosensitive. Earlier studies evaluating the success of radiation therapy showed that neurologic improvement was obtained in 40% to 50% of patients, but more recent studies have demonstrated a success rate of 70%. Pain relief may be delayed for up to 2 weeks after the start of radiotherapy, and this treatment does not correct existing biomechanical abnormalities or stabilize the spine. In addition, it is not effective for preventing imminent vertebral body collapse; almost half of the patients undergoing radiotherapy subsequently experience vertebral body compression fractures. Because of these drawbacks, adding a spine stabilization procedure to a radiation therapy program is thought to be critical for rapidly managing axial pain and for providing neurologic recovery.
Open Surgery
Historically, the indications for surgical intervention have included radioresistant disease, spinal instability, spinal cord compression, acute or progressive neurologic deterioration, previous exposure of the spinal cord to radiation, incapacitating pain (despite orthotic treatment or radiation), impending pathologic fracture, and life expectancy of at least 3 months. The principal objectives of surgery include nerve root decompression, stabilization, and reconstruction of the anatomic spinal column. Surgery is thought to maximize the patient’s quality of life because it is likely to restore or preserve neurologic function and relieve pain. Nevertheless, prospective surgical candidates must be considered adequately fit for undergoing a surgical procedure that is associated with high risk of significant complications.
The surgical approach depends on
- 1.
Location of the tumor
- 2.
Presence or absence of spinal instability and
- 3.
Presence or absence of neural compression or neural deficit.
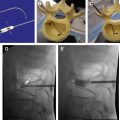
In 1989, Weinstein proposed a model designed to enhance surgical planning in patients with spinal metastasis. In this model, the vertebral body is delineated into 4 zones ( Fig. 1 ), which are used to describe location of the metastatic lesion. Zone I includes the spinous process to pars interarticularis and superior facet. Zone II encompasses the superior articular facet, transverse process, and the pedicle from the level of the pars to its junction with the vertebral body. Zone III consists of the anterior three-quarters of the vertebral body, and zone IV is the posterior one-quarter of the vertebral body. Within these zones, the tumor itself is described as intraosseous, extraosseous, or distance metastasis.
Zone I and zone II lesions are accessed using a posterior or posterolateral surgical approach. These types of lesions are usually treated using posterior decompression and stabilization. Zone III lesions are typically accessed using an anterior surgical approach; this allows direct access to the tumor, and effective reconstruction of the weight-bearing anterior portion of the spinal column can be achieved using allograft, autograft, cages, plates, or polymethylmethacrylate implants. Zone IV lesions are the most difficult to treat because they require a combined anterior and posterior approach.
Percutaneous image-guided vertebral body augmentation
In 1981, Harrington was the first to propose using bone cement augmentation as a method for relieving pain that is associated with malignant spinal tumors. In essence, the investigator described a “fusion” type of procedure, in which bone cement was inserted around and into tumor-affected vertebral bodies after removing portions of the tumor, as a method to stabilize the spine.
Subsequently, percutaneous vertebral body augmentation procedures, such as vertebroplasty and kyphoplasty, were developed as a means for managing malignant spine lesions and used with good success. During the mid-1990s, Cotten and colleagues reported that 97% of patients had at least some pain relief within the first 48 hours after undergoing a vertebroplasty procedure. Of these patients, 13.5% were pain free, 55% showed substantial improvement, and 30% were moderately improved ( Fig. 2 ). Weill and colleageus reported that 73% and 65%, respectively, of 37 patients undergoing vertebroplasty for malignant spinal lesions had sustained pain relief at 6 and 12 months postoperatively. Deramond and colleagues suggested moderate to complete pain relief in 80% of 101 patients. Using kyphoplasty in 18 patients for treating vertebral compression fractures associated with multiple myeloma, Dudeney and colleagues reported significantly improved quality of life through at least 52 months as measured using SF-36 scores. Pflugmacher and colleagues reported significantly decreased pain and significantly improved function scores in a series of 20 patients through at least 12 months. Fourney and Gokaslan reported that 84% of 56 patients, 21 with myeloma and 35 with primary spinal malignancy, had marked or complete pain relief through at least the first year.
Nevertheless, percutaneous vertebral body augmentation used for treating advanced spinal metastasis has not been widely adopted because it can be technically challenging to perform safely in this population. Most patients with metastatic lesions have epidural extension of the tumor, with possible disruption of the posterior cortical border. Complications associated with bone cement extravasation occur more often when treating patients with metastatic disease (up to 10%) than those with osteoporosis (1%–2%) or aggressive vertebral hemangiomas (2%–3%). Mousavi and colleagues reported that the risk of cement leakage during percutaneous vertebroplasty in the metastatic spine is significant, with up to 85.7% of procedures resulting in cement extravasation outside of the vertebral body. However, extravasation in these cases is not usually clinically significant. The higher risk of extravasation in patients with spinal malignancy, compared with patients with osteoporotic vertebra, may be attributed to the increased in situ pressures generated during the procedure. Injection of bone cement into a vertebral body with a resident tumor is more difficult than into an osteoporotic vertebral body. The potential for eliciting tumor cell extravasation is a concern, and it deters many clinicians from using percutaneous vertebral body augmentation as a treatment option for patients with spinal metastasis.
Clearly, percutaneous vertebral body augmentation procedures can also be used in conjunction with conventional oncology therapies, including radiation therapy; however, the potentially significant clinical drawbacks have inhibited its use. Subsequently, clinical research in this area has tended to revolve around minimizing the limitations of using conventional vertebroplasty or kyphoplasty in this application by developing new techniques for permitting a safe and efficacious procedure.
Adaptations to conventional vertebral body augmentation
Radiofrequency Ablation with Vertebroplasty
Grönemeyer and colleagues were the first to report their clinical experience using radiofrequency ablation (RFA) of spinal tumors in combination with vertebroplasty. Patients had unresectable, osteolytic spine metastases and had not responded to radiotherapy or chemotherapy. The concept of using the combination of RFA and vertebroplasty caught on quickly as a technically innovative procedure for treating patients with metastatic spinal tumors. Immediately after Grönemeyer’s report, several other investigators reported similar positive clinical outcomes in individual cases and small case series using this procedure.
A larger multicenter study (n = 43) was published in 2004. All patients had tried and failed, or were ineligible for, receiving conventional treatments such as radiotherapy or surgery for treating spinal metastases. Before RFA treatment, patients had a mean pain score of 7.9 points; by 24 weeks posttreatment, the average pain score was reduced to 1.4 points. Complications observed with the procedure included second-degree skin burn at the grounding pad site (n = 1), transient bowel and bladder incontinence after the treatment of a metastasis involving the sacrum (n = 1), and fracture of the acetabulum after RFA of an acetabular lesion (n = 1). Longer-term follow-up of the same cohort was conducted to evaluate tumor progression and postoperative complications. The mean time to tumor progression was 730 ± 54 days (Kaplan-Meier estimate).
When the posterior wall of the vertebra is compromised by the malignancy, RFA may pose a substantial risk for nerve damage because the spinal cord is in close proximity to the ablation field. Buy and colleagues reported on a modified RFA delivery method that was designed to obviate the risk of inferring radiofrequency current into the spinal cord. Three patients with high-risk spinal tumors were treated. The RFA device that was used consisted of 2 needles (electrodes) that were activated in a bed of hypertonic saline (5.85%). This design allows more control over energy delivery than the conventional monopolar RFA technology.
Cryoablation with Vertebral Body Augmentation
If the lesion extends into the paravertebral area, a choice between standard RFA and cryoablation (CRA) can be used to control the soft tissue mass before the augmentation of the vertebral body. RFA requires heavy sedation or general anesthesia, which is not the case if CRA is used. Both techniques are usually performed under computed tomographic (CT) guidance in a similar manner in other parts of the body. With CRA, an ice ball is created to ablate the soft tissue tumor. The size and location of such an ice ball can be accurately created under CT guidance, a benefit not available with RFA. After tumor ablation with RFA or CRA, the vertebral augmentation portion of the combined procedure is performed under direct visualization either by CT fluoroscopy, placing a C-arm in the CT suit, or by transferring the patient into the angiography room.
Tumor Debulking Combined with Vertebral Body Augmentation
In 2006, Tschirhart and colleagues suggested tumor-volume reduction (as opposed to tumor necrosis occurring with RFA) be used in concert with vertebroplasty as a useful means for stabilizing vertebral bodies infiltrated by metastatic tumors. Using a biphasic parametric finite element analysis (FEA) model simulating a tumor infiltrated vertebral body at the lumbar level, the investigators reported that restoration of vertebral stability was theoretically possible after removing 30% of the tumor and inserting 1 to 2 mL of bone cement. The model indicated that creation of a cavity in the vertebra would permit preferential cement deposition in the region affected by lytic disease and facilitate restoration of stability because the region of lytic destruction and the surrounding bone would both be stabilized.
The following year, Ahn and colleagues confirmed the use of Tschirhart’s FEA model in cadaver tissues. Use of laser-induced thermotherapy to ablate the tumor immediately before vertebroplasty-facilitated bone cement placement improved biomechanical stability and reduced the risk of cement extravasation over using the vertebroplasty procedure alone.
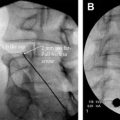
Radiofrequency-based plasma ablation has become available as a method that can also be used to create a void in vertebral bodies affected by metastasis. This type of technology allows creation of a void in the tissue, similar to the laser devices, but is recognized to be safer to use in several clinical applications. With this type of device, radiofrequency energy is used to excite the electrolytes in a conductive medium, such as saline solution, creating precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds, therefore having the ability to remove soft tissue at low temperatures, typically 40°C to 70°C. Georgy and Wong reported clinical feasibility and preliminary outcomes by using this type of device to make a void in the affected vertebral body before inserting bone cement. With this procedure, they found that it was possible to perform percutaneous cement augmentation in patients with advanced malignancy who would not normally be considered good candidates for undergoing conventional vertebroplasty or kyphoplasty. This technique was found to have the dual benefit of stabilizing the anterior column while minimizing the risk of anterior corpectomy by placing the injected cement in the anterior vertebral body, away from the compromised posterior wall ( Fig. 3 ).