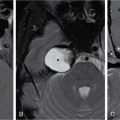
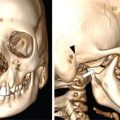
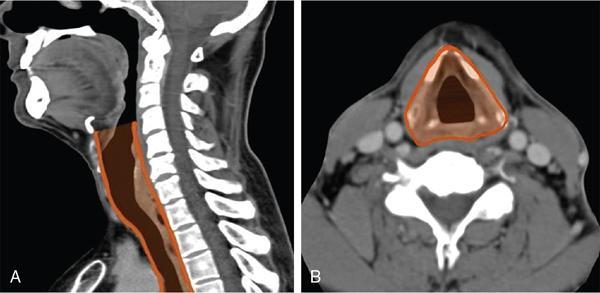
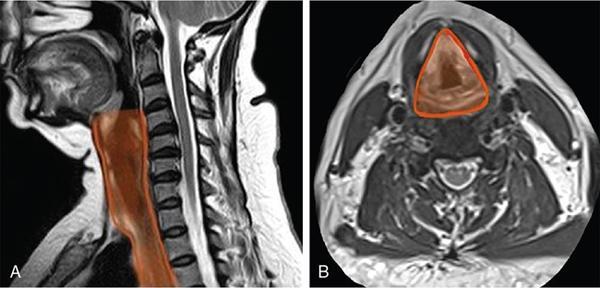
Seetharaman C, N. Sumathi, N. Meena, K. Gobi LEARNING OBJECTIVES KEY POINTS The visceral space (VS) is a central tubular space extending from the hyoid to the mediastinum and enclosed by the middle layer of the deep cervical fascia. Visceral space is confined and unique to infrahyoid neck region and contains the viscera in the anterior aspect of the neck. Visceral space extends from the hyoid bone level up to the superior mediastinum. The visceral space is flanked on either side by carotid space (CS). Visceral space is enclosed by the middle (visceral) layer of the deep cervical fascia (MLDCF). It is the most arbitrarily described of all the fascial layers of the neck. Some authors believe that the MLDCF is further subdivided into three layers. The middle layer of MLDCF splits to encase the strap muscles and the deeper layer is closely applied to the outer surface of the pharynx and oesophagus. The space contained within this fascial layer, along with its contents has a minimal quantity of adipose tissue in it. This is seen between the outer visceral fascia and those covering the deeper strap muscles anteriorly, the carotid sheaths laterally (all layers of the DCF contribute to this sheath), and the alar fascia (DLDCF) dorsally. At the level of T1 vertebra, the inferior thyroid artery pierces the MLDCF. It carries with it a fascia that longitudinally divides the visceral space into two compartments, the anterior pre tracheal space and the posterior retro-oesophagal space inferiorly. This is the reason, why thyroid lesions tend to extend into the anterior mediastinum and lesions along the oesophagus extend into the middle mediastinum. The visceral layer encompasses the larynx, trachea, hypopharynx, cervical oesophagus, thyroid and parathyroid glands, the visceral (level VI) nodes, recurrent laryngeal nerves, and the loose connective tissue that surrounds these viscera. Recurrent laryngeal nerves: The recurrent laryngeal nerves are branches of the vagus nerve. The recurrent laryngeal nerves are seen coursing along the tracheo-oesophagal groove, forming a loop with respect to the right subclavian artery and through the left aortopulmonary window. It provides motor innervation to all laryngeal muscles except the cricothyroid muscle. Level VI nodes: Anterior compartment nodes form level VI nodes and include anterior jugular, pre-tracheal, paratracheal, pre-cricoid (Delphian) and perithyroid nodes. The superior margin is the lower border of the hyoid bone, inferiorly up to the suprasternal notch and laterally up to medial limits of carotid arteries (Figs. 3.12.1 and 3.12.2). At 25 days gestation, a median diverticulum arises from the ventral wall of the foregut between IV and VI pharyngeal arches (V is rudimentary), which subsequently leads to the development of the respiratory tract, consisting of structures from both endoderm and mesoderm. The trachea-oesophagal septum divides the diverticulum in the craniocaudal direction with the anterior portion developing into trachea and the oesophagus posteriorly. This section will focus on the cervical parts of the trachea and oesophagus. The remaining structures of the visceral space are discussed in detail in other specific chapters. The oesophagus is a muscular tube-like organ located between the lower border of the laryngeal part of the pharynx and the cardia of the stomach, corresponding to the C6 vertebra proximally and T11 vertebra in the distal point topographically. The oesophagus consists of four histologic layers: mucosa, submucosa, muscularis propia, and adventitia. The mucosa is lined by non-keratinized stratified squamous epithelium. The submucosa contains elastic and collagen fibres forming irregular connective tissue and consists of veins, lymphatics and Meissner plexus. Muscularis propria contains both longitudinal (superficial) and circular muscle (deep) fibres. Longitudinal fibres begin from the posterior face of the cricoid cartilage and form ‘Lamier triangle’ limited by longitudinal muscle fibres laterally and cricopharyngeus muscle superiorly. ‘Killian triangle,’ is also found in this area bordered by the inferior constrictor muscle of the pharynx and cricopharyngeus muscle. These weaker areas give rise to Zencker’s diverticula. Circular muscle fibres are more elliptical in their upper third while becoming more circular in the lower third part of the oesophagus. They run in an irregular pattern making a shutter-like system. The upper quarter of the oesophagus consists of striated muscle, the second quarter consists of both striated and smooth muscle fibres and the distal half consists of smooth muscle fibres. Adventitia surrounds most of the oesophagus. Because of the absence of serosa, infections and tumours can spread easily to the adjacent spaces. The cervical oesophagus is 5–6 cm long and ends at the level of inferior edge of T1 vertebra. The luminal diameter is 1.4–1.5 cm at its narrowest. Cervical oesophagus has two narrow points, one classical at its beginning and the other one transitional is at superior border of sternum. The cervical oesophagus is supplied primarily by the inferior thyroid artery. Other supplying vessels include the subclavian artery, common carotid artery, vertebral, ascending pharyngeal arteries, and superficial cervical and costo-cervical trunk. The venous system of the cervical oesophagus begins at the submucosal plexus and traverses the muscular layer and empties into inferior thyroid veins. Parasympathetic fibres are supplied by the bilateral recurrent laryngeal nerves. Sympathetic nerves take origin from the pharyngeal plexus formed by upper and middle cervical ganglions and sympathetic trunks. The lymphatic drainage of the cervical oesophagus passes to pre- and para-tracheal lymph nodes (level VI), eventually draining into the lower jugular nodes (level IV). The trachea is a cartilaginous tubular structure connecting the larynx superiorly and the main bronchi inferiorly, extending from the lower edge of the cricoid cartilage (C6 level) to the carina (T4 level). Internally, the trachea has 18 to 22 D-shaped “rings”. It has anterior and lateral walls made of C-shaped hyaline cartilage while the posterior membranous wall connects the arms of the cartilage. The Trachealis muscle is seen in close proximity to the oesophagus while it runs posteriorly. A membranous connection exists between the inferior edge of the upper cartilage to the superior edge of the lower cartilage. The trachea maintains its integrity in anterior and lateral aspects due to cartilage rings. The posterior membranous wall is soft and shows contour change during swallowing, coughing and forced expiration. Trachea measures approx. 11.8 cm in length (range of 10–13 cm) in males. It tends to be shorter in females. Overall, other dimensions including diameter depend on height, age, and gender. In men, it ranges from 13 to 25 mm in the coronal plane and 13–27 mm in the sagittal plane. Its diameter tends to be lesser in women, ranging from 10 to 21 mm in the coronal plane and 10–23 mm in the sagittal plane. But generally, the tracheal diameter in the first year of life does not exceed 4 mm. In childhood, its diameter is approximately equal to the age in years. Cervical tracheal cartilages are supplied by lateral tracheal vascular pedicles, derived from branches of the inferior thyroid arteries. The arteries run along with lateral aspects of the trachea and branch off in every inter-cartilaginous ligament to supply cartilage and mucosa. These segmental arteries form anastomosis with superior and inferior segmental arteries as well as with the other half vessels. The posterior wall is supplied by branches from oesophagal arteries. Bilateral recurrent laryngeal nerves ascend along the trachea-oesophagal groove on either side after branching off from vagus nerves. These nerves supply parasympathetic (secretory glands), sensation and motor innervation to the trachea. Sympathetic nerves are from the middle cervical ganglion and are responsible for bronchodilation and reduced secretions. The lymphatic drainage from the proximal two-thirds of the trachea passes to pre- and para-tracheal lymph nodes (level VI), eventually draining into the lower jugular nodes (level IV). Based on the anatomical knowledge of DCF and the contents of visceral space, it is easier to localize the lesions to the organ of origin. In general, when a visceral space lesion is malignant (i.e., invasive appearing), the only clue to its visceral space origin is the loss of soft tissue planes with adjacent structures of the visceral space (larynx, trachea, oesophagus, thyroid). Currently, the imaging modalities available for imaging of trachea, oesophagus and its pathologies include plain X-rays, barium oesophagography, USG, CT, MRI and PET-CT. With its ability for faster acquisition and reconstruction capabilities CT is the primary imaging modality of choice. Neck and chest radiographs are usually employed as an initial screening modality to assess the presence, location, shape, and size of a radiopaque foreign body or unknown shaped objects as well as possible signs of perforation seen as extra-luminal air. Biplanar radiography reduces the false-negative rate, which is up to 47%. Tracheobronchial and oesophagal foreign bodies can be differentiated on the lateral projection. However, the false-negative rates can be as high as 85% for evaluating food bolus impaction, thin metal objects, wood and plastic objects, glass fragments, and fish or chicken bones on X-rays. The cervical portion of the trachea can be visualized on a routine chest PA view and lateral views. The lateral neck projection is obtained with the head slightly hyperextended, which is useful for studying the cervical trachea. Bilateral oblique chest X-rays help to avoid the overlapping mediastinal shadows and to view the mediastinal trachea. The high kilovoltage technique (140 kV) improves the visualization of tracheal abnormalities. To avoid buckling of the tracheal wall during expiration, especially in children the images are acquired at maximal inspiration. On conventional radiographs, the tracheal wall (1–2 mm) can be identified by air in the lumen as well its contrast with the adjacent mediastinal fat. Imaging studies using barium are useful as an initial imaging modality for evaluating patients with dysphagia, reflux, motility disorders, or perforation. One must be careful in patients with suspected perforation, wherein a diluted water-soluble contrast agent is typically used, and switch over to diluted barium when no perforation is detected. Thick barium can be used for patients with an intact oesophagus. Barium studies are not used in the evaluation of trachea or bronchi. The advent of MDCT has revolutionized imaging. With a drastic reduction of acquisition time, CT of the neck and chest produce images of diagnostic quality that can be obtained in a few seconds even in patients with breathlessness. CT is the most used modality for evaluating cervical oesophagal pathology, including assessing mass lesions and their extent, suspected foreign bodies, oesophagal wall perforation as well as evaluation of adjacent structures. CECT is indicated to assess mucosa and mass lesions. CT plays an essential role in evaluating the various features of early and advanced carcinoma oesophagus, its local extensions, and metastasis, thus helping the surgeons to plan the best line of management. CT is also used for planning radiation therapy treatment. Normally, distended oesophagal wall thickness is less than 3 mm and thickness greater than 5 mm is usually abnormal. Early stages of oesophagal cancer are seen as asymmetric wall thickening or intraluminal mass. Invasion through adventitia into the adjacent fat planes is seen as ill-defined soft tissue around the mass lesion. A diluted water-soluble contrast agent is used orally in the evaluation of oesophagal lumen, suspected perforation, and evaluation of the integrity of the anastomosis. CT is extremely useful to identify foreign body impaction and has a sensitivity and specificity of above 90%–100% in evaluation of symptomatic fish bones. CT scan is necessary in evaluation of perforation and its related compilations (abscess, mediastinitis and fistula). CT is the modality of choice for imaging of trachea and bronchi. For imaging of the trachea, the acquisition is usually done in end- inspiratory phase. For conditions like tracheomalacia, it is done with inspiratory and expiratory phases to assess the collapse of the trachea, inward bowing of the posterior membranous portion and decreased AP diameter by 50% or greater. Axial and reconstructed coronal and sagittal images are useful in studying specific details of lesions within or outside the tracheal lumen, their relation to the tracheal wall and surrounding structures, features of invasion and mass effect. Contrast-enhanced CT helps to analyze vascular structures, study enhancement patterns of lesions and for performing CT angiography. Various 3D reconstruction techniques aid in improving diagnosis – minimum intensity projection (MIP), 3D shaded surface display (SSD), multiplanar volume reconstruction (MPVR), and volume-rendering (VR) techniques. Virtual bronchoscopy (VB): Viewing the interior of the trachea and the airway structures in VR images is called virtual bronchoscopy. It helps us to identify subtle lesions, the extent of the lesion in cases of significant stenosis where a conventional scope cannot be passed beyond the stenosis. It can also act as a roadmap before performing conventional bronchoscopy guided procedures. With its longer acquisition time, MRI was not preferred in the initial days. However, with the newer advanced short acquisition time sequences, MRI is increasingly being used in the imaging of neck abnormalities including trachea and oesophagus. Even though the role of MRI is limited in imaging the trachea and cervical oesophagus, its non-ionizing radiation nature makes it appealing for evaluation of young children and adolescents who may require frequent imaging. With its inherent soft tissue characterization, it scores over CT in the local and regional staging of tumours. Though ultrasound is not routinely used for the evaluation of tracheal and oesophagal pathologies, it can be used in critical care set-up and in operation theatres. The advantages are that it is available at the bedside and does not need the complete immobility of the patient. To obtain a detailed visualization of the oesophagal layers, endoscopic ultrasound is used. Also, one can biopsy any suspicious lesions and adjacent lymphadenopathy if present. Endoscopic ultrasound can delineate all the layers of the oesophagus, including, However, the endoscopic ultrasound is more useful in evaluating thoracic oesophagal abnormalities. Transcutaneous oesophagal ultrasound is useful in evaluating infiltration of cervical oesophagus by other cancers in its vicinities such as thyroid, parathyroid, trachea and nodal masses. In addition, ultrasound is useful in the regional staging of oesophagal cancer, particularly in identifying and confirming the nature of any associated neck nodes. Conventional ultrasound can be used in children for evaluation of cervical oesophagus through right and left lateral approach. The cervical oesophagus is a mobile structure. On ultrasound, when the patient’s head is turned to the left, the oesophagus moves to the right and when the patient’s head is turned right, the oesophagus moves to the left. The cartilages appear as sonolucent areas. The anterior interface between the mucosa and the air column appears as a hyperechoic line followed by reverberation artefacts. Tracheal cartilage in a longitudinal plane is seen as a ‘string of beads’ and inverted U in the transverse plane. Ultrasound has been proven to be a good modality for measuring the internal diameter of the trachea and sizing of the endotracheal tube accordingly. Ultrasound can predict difficult laryngoscopy by using a few parameters like inability to visualize hyoid bone, reduced hyomental distance ratio (1–1.05), increased anterior neck thickness (>2.8 cm) and increased tongue thickness (>6.1 cm). Ultrasound is useful for confirming the position of the endotracheal (ET) tube and laryngeal mask airway (LMA). Upper airway ultrasound is also useful for doing percutaneous cricothyroidotomy and percutaneous dilational tracheostomy (PDT), significantly reducing procedure-related complications (Fig. 3.12.3). FDG-PET imaging is typically reserved for cases to delineate between malignant and nonmalignant etiologies and to stage oesophagal and tracheobronchial malignancies. It is also useful in post-treatment surveillance to identify any local, regional, or distant disease/recurrence) (Table 3.12.1). Modified from T. Marini, A. Desai, K. Kaproth-Joslin, J. Wandtke, S.K. Hobbs. Imaging of the oesophagus: beyond cancer. Insights Imag. 8 (3) (June 2017) 365–376. Luminal pathology of the oesophagus can be due to disorders of smooth muscle innervation, scarring, and foreign body impaction. Dilatation of cervical oesophagus with a diameter of >10 mm and containing air-fluid level should be considered abnormal. A rough estimate of the luminal size of oesophagus is given by comparing it with the adjacent trachea and is considered abnormal if at least a third of oesophagus starts approaching the tracheal size. Further evaluation is warranted with other relevant imaging modalities. A variety of processes affecting the upper aero digestive tract may result in cervical oesophagal stricture, including malignancy, caustic ingestion, epidermolysis bullosa, previous surgery, radiation therapy or traumatic injury.
3.12: Visceral space (cervical oesophagus and trachea)
Abbreviations (in order of appearance)
Introduction
Extent, fascial anatomy, and relations
Contents
Embryology of trachea and oesophagus
Imaging anatomy of the cervical oesophagus
Structure of oesophagus
Blood supply
Nerve supply and lymphatic drainage
Imaging anatomy of trachea
Structure of trachea
Blood supply
Nerve supply and lymphatic drainage
Imaging approach for localization of lesions in visceral space
Imaging modalities
X-ray
Oesophagus
Trachea
Barium studies
Oesophagus
Trachea
CT
Oesophagus
Trachea
MRI
Ultrasound
Oesophagus
Trachea
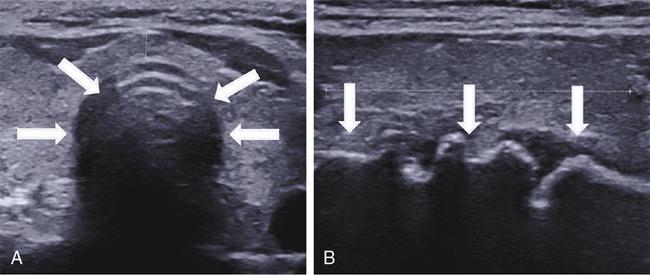
Sonoanatomy of upper airway
FDG-PET
Luminal Abnormalities
Wall Abnormalities
Extra Luminal
Benign Neoplasms
Malignant Neoplasms
Non-neoplastic abnormalities of cervical oesophagus
Luminal disorders
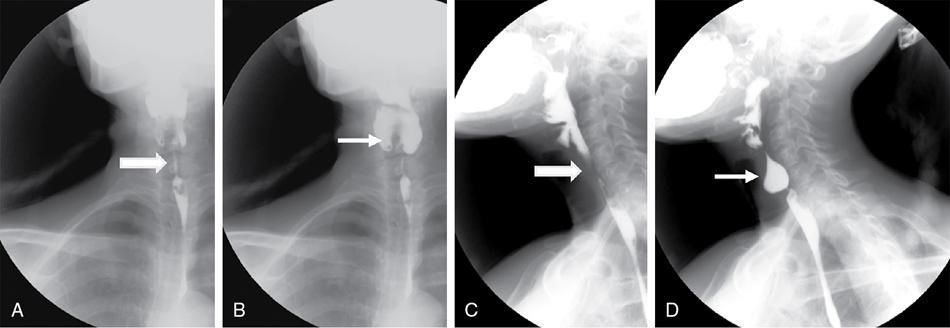
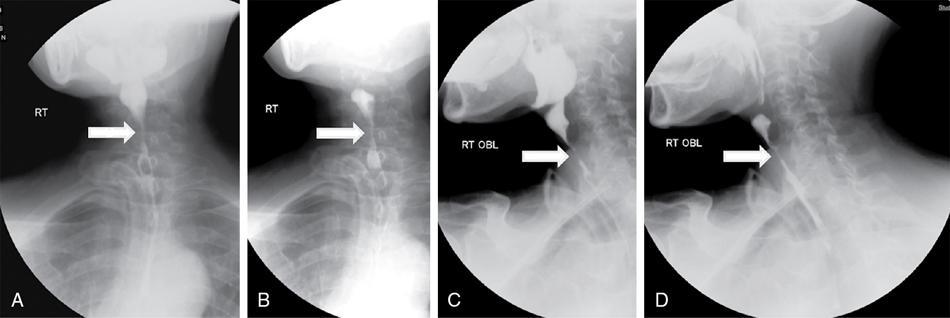
Stricture
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree