James K. Min, Daniel S. Berman and Jonathon Leipsic (eds.)Multimodality Imaging for Transcatheter Aortic Valve Replacement201410.1007/978-1-4471-2798-7_38
© Springer-Verlag London 2014
38. What Future Studies Are Needed for TAVR Imaging?
(1)
Weill Cornell Medical College, NewYork-Presbyterian Hospital, New York, NY, USA
(2)
Division of Cardiology, Department of Radiology, University of British Columbia, St. Paul’s Hospital, Vancouver, BC, Canada
(3)
Departments of Cardiovascular Medicine and Radiology, Imaging Institute and Heart and Vascular Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA
Abstract
The development of transcatheter valve procedures has been revealed an ever-increasing importance of pre-, peri-, and post-procedural imaging. Extensive imaging evaluation of the aortic valve, aortic root, aorta, and iliac arteries has contributed to the evolution and optimization the procedure. The initial approach has been focused towards obtaining a maximum amount of information, often times with multiple imaging modalities. Eventually, it will be necessary to consolidate the accumulating data into standardized imaging approaches, which can be recommended only to operators in the presence of high-quality scientific evidence.
Keywords
Aortic valveTranscatheter heart valveStenosisPost-procedural evaluationProcedural simulationStrokeIntroduction
The development of transcatheter valve procedures has been revealed an ever-increasing importance of pre-, peri-, and post-procedural imaging. Extensive imaging evaluation of the aortic valve, aortic root, aorta, and iliac arteries has contributed to the evolution and optimization the procedure. The initial approach has been focused towards obtaining a maximum amount of information, often times with multiple imaging modalities. Eventually, it will be necessary to consolidate the accumulating data into standardized imaging approaches, which can be recommended only to operators in the presence of high-quality scientific evidence. Following the other chapter in this book, which describe the current status of the transcatheter aortic valve replacement (TAVR) experience, the goal of this chapter is to consider what future imaging clinical research studies are most needed to provide the basis for evidence-based consensus guidelines.
Annulus and Aortic Valve
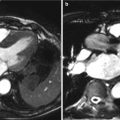
Optimal adaptation of the aortic valve prosthesis to the aortic annulus is a prerequisite for long-term durability of the transcatheter valve. During surgical aortic valve replacement, the size of the annulus is measured with dilaters, matching common size valve prostheses. After choosing the appropriate size valve, suturing the valve ring to the aortic annulus provides tight circumferential adaptation. In contrast, during TAVR, the stent anchors and adapts to the anatomy of the aortic annulus and root. This process is dependent on details of deployment, size, geometry, and tissue composition of the landing zone. Therefore, as describe in other chapters of this book, assessment of the complex anatomy of the aortic annulus (virtual basal ring), aortic valve, and aortic root is critical for successful TAVR. Anatomically, the aortic valve leaflets are attached to the aortic root in a crown-shaped manner along the commissures and often referred to as a coronet [1]. By imaging, the annulus (or better inferior virtual basal ring) is defined by the level of basal attachment of the leaflets.
Multiple studies have examined differences in aortic annulus measurements between echocardiography and multidetector row computed tomography (MDCT) and have demonstrated an ovoid shape of the annulus with significant differences in diameters between modalities [2–7]. The mean MDCT diameter is significantly larger than the echocardiographic measurements, although the differences are small. Current clinical experience and recommendations are based on echocardiographic measurements. Using a decision model for the TAVR strategy with the TEE measurement as the gold standard, Messika-Zeitoun et al. [2] demonstrated that more patients would have been denied the procedure based on the MDCT measurements, despite good results with the standard TEE-based strategy. These data demonstrate that the increased precision of three-dimensional measurements with MDCT do not necessarily translate to improved clinical outcome without the appropriate development of MDCT transcatheter heart valve sizing guidelines. These limitations serve to define the need for future studies correlating imaging findings with clinical outcome.
The distance between the annulus and the ostia of the coronary arteries in relationship to the length of the coronary leaflets can be derived from MDCT scans [3–7]. In a small minority of patients, a particularly low origin (<10 mm) has been associated with coronary complication during the procedure. However, based on current experience, this is an infrequent event and careful assessment during routine cardiac catheterization or on standard MDCT scans can provide reliable information. While some studies suggest remodeling of the root including the origin of the coronary arteries in patients with AS, it is unlikely that more detailed description will have significant clinical impact.
What Future Studies Are Needed to Assess the Annulus and Valve?
Previously published data demonstrate that the increased precision of three-dimensional measurements by MDCT do not automatically correspond with better clinical outcome and define the need for future comparative studies correlating imaging findings with clinical outcome. Areas where data is still incomplete and future studies appear necessary are manifold.
Appropriate device sizing is most likely dependent on not only pre-procedural dimensions but also tissue composition and distribution of disease. As the hinge points of the valve leaflets provide the anchor point to the valve, differences in the characteristics of the sub-leaflet tissue (muscular septum, membranous septum, or aortomitral curtain) will determine distortion at the time of stent valve deployment. Understanding how an aortic valve prosthesis interacts with the aortic root anatomy and the left ventricular outflow tract will allow prediction the final shape of the prosthetic valve, displacement of the native calcified leaflets, and sealing around the prosthesis [8]. Of particular interest is the extent and distribution of the aortic valve and root calcification, including extension of calcification along the LVOT [9, 10] and mitral annular calcification (MAC). This information may facilitate proper placement and subsequent aortic regurgitation, dysfunction of the conduction system, and mitral valve function.
The impact of more detailed assessment of the aortic annulus and root requires examination in future clinical outcome trials. In such studies, echocardiographic outcomes should be compared to other modalities for measures of post-procedural aortic regurgitation and other imaging complications. Clinical endpoints should invariably be assessed as well and include conduction abnormalities (with subsequent need for permanent pacemaker implantation), morbidity, and mortality. Such data will need to be derived from emerging large registries and eventually from prospective trials.
Discordance of Aortic Stenosis Severity
Grading of AS with echocardiography is well standardized for the majority of patients. However, there is a non-negligible portion of patients who exhibit discrepancies between aortic valve area (AVA) and transvalvular gradient for measures of AS severity. In fact, for as many as 30 % of patients with a calculated AVA in the severe range, other parameters suggest only mild or moderate disease (e.g., mean gradient 30 mmHg) [11]. These patients with low-flow low-gradient AS (LF-LGAS) may truly have severe AS with resultant myocardial failure (true AS) or may have more moderate degrees of AS and unrelated myocardial dysfunction (pseudo-AS). In the latter setting, the aortic valve may appear severely stenotic as a result of the flow-dependent nature of the valve area calculation by either invasive or noninvasive techniques [12] and the inability of the myopathic ventricle to generate adequate force to fully open the valve. Distinguishing between these possibilities has important clinical implications with regard to prognosis and management options, as patients with true AS will likely benefit from corrective valve surgery, whereas patients with pseudo-AS may not.
While the majority of patients with LF-LGAS have reduced left ventricular systolic function (LVEF <40 %), a number of patients have paradoxically low flow, defined as a stroke volume index of 35 mL/m2, despite preserved LVEF >50 % [13]. These patients have lower peak transaortic velocities (3.5 vs. 4.0 m/s) and lower mean gradients (32 vs. 40 mmHg) compared to patients who have normal transaortic flow rates, despite having similar AVA (0.76 vs. 0.84 cm2), similar dimensionless index (0.24 vs. 0.23), and similar LVEF (65 % vs. 69 %). These features predispose diagnosis to an underestimation of the severity of AS in these patients and delay in pursuing appropriate care. The mechanism of the paradoxically low flow in the face of preserved LVEF likely relates to high afterload, and the reduced stroke volume in this setting is probably an early marker of intrinsic myocardial dysfunction.
The prognosis of patients with LF-LGAS with preserved LVEF depends, in part, on the presence of symptoms [11, 13]. In a series of symptomatic patients, those with low transaortic flow experienced a worse prognosis compared with their counterparts with normal flow (3-year survival 76 % vs. 86 %); however, they obtained similar mortality benefits from AVR [13]. In contrast, in a larger and more contemporary report, asymptomatic patients with LF-LGAS with preserved LVEF had a prognosis similar to that of patients with more moderate AS (92.2 % vs. 95.1 % survival at 46 months) [11].
Determining which patients with LF-LGAS will benefit from AVR can be challenging [14–17]. The utility of a dobutamine challenge in the evaluation of LF-LGAS during either cardiac catheterization or stress echocardiography has been well documented [18–22], but differentiation between true from pseudo-AS in patients with no contractile reserve remains challenging.
What Future Studies Are Necessaryin AS Grading?
Other imaging modalities may aid in the identification of severe AS in this setting. Recent small studies have demonstrated good correlation between the aortic valve calcium score as measured by multidetector computed tomography and the severity of AS as assessed by echocardiography [23, 24].
Another study correlated delayed enhancement by preoperative cardiac magnetic resonance (MR) with the presence of myocardial fibrosis on surgical biopsy in patients with severe AS [25, 26]. Although the utility of MR for determining the prognosis of patients with LF-LGAS is unclear, it is likely that the lack of contractile reserve in some patients with LF-LGAS is mediated, in part, by the presence of irreversible cardiac fibrosis. While these modalities may help to further risk stratify these patients, data is incomplete and correlation with clinical outcome is missing. Large registries and future randomized studies will provide answers.
Stroke, Aorta, and Plaque
A concern based on the initial randomized trials of TAVR is the higher initial stroke rate with TAVR. In the PARTNER trial [27] at 1 year, the rate of death from any cause was 30.7 % with TAVR, as compared with 50.7 % with standard therapy (hazard ratio with TAVR, 0.55; 95 % confidence interval [CI], 0.40 to 0.74; p < 0.001). However, at 30 days, TAVR—when compared with standard therapy—was associated with a higher incidence of major strokes (5.0 % vs. 1.1 %, p = 0.06). Randomized trials have also identified a higher incidence of CVI with TAVR compared to surgical treatment [28]. A recent study described a prospective investigation of silent and clinically apparent cerebral embolic events and neurological impairment after TAVR [29]. The authors investigated peri-interventional cerebral embolism with diffusion-weighted magnetic resonance imaging (DW-MRI) and its relationship to clinical and serologic parameters of brain injury. Cerebral DW-MRI was performed before, directly, and 3 months after TAVR with the current third-generation self-expanding CoreValve (Medtronic, Minneapolis, Minnesota) prosthesis. At the time points of these serial MRI studies, focal neurological impairment was assessed according to the National Institutes of Health Stroke Scale (NIHSS), and serum concentration of neuron-specific enolase (NSE), a marker of the volume of brain tissue involved in an ischemic event, was determined. Thirty patients were enrolled and 22 completed the imaging protocol. Three patients (10 %) had new neurological findings after TAVR, of whom only 1 (3.6 %) had a permanent neurological impairment. Of the 22 TAVR patients with complete imaging data, 16 (72.7 %) had 75 new cerebral lesions after TAVR presumed to be embolic. The NIHSS and NSE were not correlated with DW-MRI lesions. While the incidence of clinically silent peri-interventional cerebral embolic lesions after TAVR was high, the incidence of persistent neurological impairment was low.
It remains unclear if stroke rates continue to remain high in the early and intermediate-term period after TAVR, and, consequently, the optimal dose and duration of anticoagulation in the post-TAVR period is unclear. A recent study examined whether there exists a high-risk period for cerebrovascular events (CVE) after TAVR [30]. Patients who underwent TAVR were evaluated at baseline, at discharge, at 1 and 6 months, and yearly. Outcomes assessed were CVE events and death. The timing of such events, predictors, and impact on survival were analyzed in a total of 253 patients with a median age was 85 years. During a follow-up of 455 days, 23 patients experienced a CVE event. The incidence was highest in the first 24 h but remained high for 2 months. In-hospital mortality rate after a CVE event was 21 %. A prior history of CVE disease was an independent predictor of an event (hazard ratio, 4.23; 95 % CI, 1.60–11.11; p = 0.004).
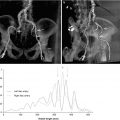
The pathophysiologic mechanisms underlying the increased CVE event rate remains incompletely understood, but aortic plaque burden may play a significant role. In population-based studies of asymptomatic individuals and persons with suspected coronary artery disease, calcified aortic atheroma has been shown to be associated with increased risk of major cardiovascular events [31, 32]. In population-based asymptomatic individuals, based on non-contrast enhanced CT studies, there is a relationship between calcified aortic atheroma [33] and all-cause mortality, independent of conventional CVD risk factors [34, 35]. In patients evaluated for conventional cardiothoracic surgery, calcified and non-calcified aortic atheromas have also been established as a predictor of postoperative complications, as well as of adverse long-term outcome [36–40].
One explanation for the role of aortic plaque invokes a dominant role of embolic complications, in particular, in the context of manipulations of the aorta during the procedure. An alternative hypothesis suggests a more systemic effect of the diffuse atherosclerotic disease process, with aortic plaque burden a marker of atherosclerotic disease and complications in other vascular regions. Importantly, the relationship of plaque extent to mortality is independent of distribution along the aorta, which is evidence that mortality is related to the systemic atherosclerotic disease process rather than focal or embolic complications alone [40]. This is further supported by recent studies in the carotid circulation and previous data describing a relationship between coronary and aortic calcifications [41]. Recent data from patients evaluated for transcatheter aortic valve implantation (TAVR), a population with very high mortality, reveal extensive atherosclerotic disease of the aorta [42]. Together, these results identify the extent of thoracic atheroma burden as a risk factor for cerebral complications of TAVR.
What Future Studies Are Needed?
Based on these data, studies examining aortic plaque burden and composition in a volumetric fashion in relationship to CVE are necessary. Such studies should employ imaging endpoints such as intracranial Doppler, MDCT, and diffuse weighted MRI along with clinical endpoints.
Vascular Access Site
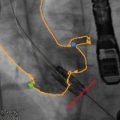
There is a spectrum of arterial access strategies for TAVR, ranging from a percutaneous transfemoral, transapical, transaxillary, to open iliofemoral approaches [43–47]. Because of the large device size of current generation systems, the transfemoral approach requires favorable iliofemoral arterial anatomy [48]. In the setting of insufficient luminal diameter, extensive calcification, or excess tortuosity of the iliofemoral arteries, the transfemoral approach is associated with a higher incidence of arterial injury [49]. The prevalence of significant peripheral arterial disease (PAD) in the high-risk population currently undergoing assessment for TAVR is high. Limited angiographic assessment of the iliofemoral anatomy is routinely performed during pre-procedural coronary angiography. However, dedicated three-dimensional evaluation of infrarenal aortic and iliofemoral arterial anatomy by MDCT provided more precise cross-sectional diameter measurements, assessment of the extent and distribution of calcification, and three-dimensional course and tortuosity of the iliofemoral arteries. This may allow identification of patients with unsuitable iliofemoral anatomy for a transfemoral approach to TAVR and eventually pre-procedural planning of arterial access [42]. Using MDCT of the infrarenal abdominal aorta and iliofemoral arteries, our group described [42] that one third of patients with severe aortic stenosis evaluated for TAVR had anatomic criteria of unfavorable atherosclerotic iliofemoral disease. Of these patients, a majority (77 %) demonstrated small luminal narrowing (<8 mm) of the iliofemoral arteries; the remaining 23 % showed other criteria of advanced disease, including circumferential calcification and severe tortuosity, despite sufficient luminal diameter. Comparison between the groups of patients with and without any of these criteria demonstrated that the presence of any one of these criteria identifies a high-risk group of patient with significantly higher clinical risk profile and more unfavorable anatomy of the entire infrarenal and iliofemoral arteries. Because of the known overlap of etiology and risk factors for atherosclerotic vascular disease and aortic stenosis, it is not surprising to find a high number of patients with significant peripheral arterial disease in this population.
Pre-procedural interventional planning is widely established and routinely performed in the context of aortic endovascular stent procedures [50, 51]. The importance for planning of vascular access may diminish with the development of TAVR valve delivery systems with small diameters. However, previous data suggest that the identification of unfavorable iliofemoral anatomy may identify a high-risk group of patients in whom careful planning of access strategy may be of particular importance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree