Musculoskeletal Constitutional Disorders
Teresa Chapman, MD
LEARNING OBJECTIVES
1. Name an ossification center that is useful in determining likelihood of a neonate being full term.
2. Describe the curvature morphology that distinguishes these types of scoliosis: idiopathic, congenital, and neuromuscular.
3. List a common condition associated with coxa valga.
4. Indicate the primary objective of radiographic evaluation of the toddler presenting with genu varus.
5. Recognize hindfoot malalignment likely to be associated with tarsal coalition.
6. Identify the two joints most commonly involved in tarsal coalition, and name an associated radiographic finding for each.
7. Describe one typical radiographic feature of the skull, the spine, the pelvis, and the extremity for each of the following three skeletal dysplasias: achondroplasia, mucopolysaccharidosis, and osteogenesis imperfecta.
INTRODUCTION
Growth of the pediatric skeleton is a complex topic that encompasses neurologic, muscular, and bone abnormalities arising from genetic anomalies, fetal growth disturbances, nutritional deficiencies, and other acquired insults such as trauma and cancer therapies. An in-depth understanding of musculoskeletal development and disease is beyond the scope of this chapter. However, the general radiologist encountering an abnormal pediatric musculoskeletal imaging study requires an essential understanding of skeletal growth, commonly seen alignment disorders, skeletal dysplasias. These categories of skeletal health will be discussed within this chapter, focusing on radiographic diagnosis and appropriate guidance of further workup and therapies.
SKELETAL GROWTH
The embryonal cartilaginous appendicular skeleton forms from mesoderm in the 4th to 8th weeks, and the skeleton gradually matures by deposition of calcium in the cartilaginous matrix of primary ossification centers. In tubular bones, calcification begins in the middle aspect of the diaphysis from the 8th to 12th weeks.2,3 The zones of proliferative calcification and the epiphyseal cartilage plate (the physis) are disclike regions that develop between the diaphysis and epiphyseal cartilage. At birth, the diaphyses of the limb bones are completely ossified, whereas the epiphyses are still cartilaginous. After birth, secondary ossification centers develop within the epiphyses, which gradually ossify at variable ages.2,3
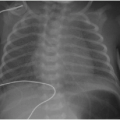
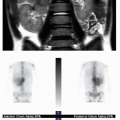
Observation of the timing and morphology of the secondary ossification centers indicates age-appropriate growth of the skeleton. It is standard practice to refer to published texts documenting expected appearances of normal secondary ossification centers.3 Understanding normal skeletal development is useful in several routine radiographic assessments, which will be briefly mentioned here. In the newborn, the proximal humeral head ossification center is visualized between 37 weeks of gestational age and 16 weeks of postnatal life, whereas visualization of the humeral head ossification center prior to 37 weeks of gestational age is very rare.4 Therefore, if the humeral head ossification centers are observed at birth (Fig. 28.1), then it can be inferred that the infant is at or near term. In the developing child presenting with elbow pain and trauma, the ossification centers of the elbow must be accounted for in the effort to exclude dislocation.5 Prediction of the elbow ossification centers is estimated using the pneumonic, CRITOE, wherein each letter represents the progressive appearance of each ossification center every 2 years, beginning at age 1 or age 2 (see Chapter 25).6 As discussed later in this chapter, the Risser stage of iliac crest apophyseal ossification becomes a relevant marker for skeletal maturity in the context of scoliosis.
Comparison to normative standards published in bone age atlases allows the radiologist to determine if the pediatric skeleton is growing normally. Common indications for bone age assessment include abnormal growth (short stature or abnormally fast growth trajectory) and either delayed or advanced pubertal development. The bones of the hand and wrist are evaluated and compared to standard images,7 with normal growth falling into a wide spectrum encompassing two standard deviations with respect to the
chronologic age (Fig. 28.2). The distal bones develop most rapidly, and therefore, common practice relies on studying the size and morphology of the metaphyses and epiphyses of the middle and distal phalanges of the fingers. The sesamoid bone of the thumb is also a reliable marker, appearing at approximately 10 years in females and 13 years in males.7 Because of the relatively slow rate of changes in the hand and wrist in the infant and toddler, evaluation of the knee or foot may be more helpful in children younger than 2 years.8,9
chronologic age (Fig. 28.2). The distal bones develop most rapidly, and therefore, common practice relies on studying the size and morphology of the metaphyses and epiphyses of the middle and distal phalanges of the fingers. The sesamoid bone of the thumb is also a reliable marker, appearing at approximately 10 years in females and 13 years in males.7 Because of the relatively slow rate of changes in the hand and wrist in the infant and toddler, evaluation of the knee or foot may be more helpful in children younger than 2 years.8,9
SKELETAL MINERALIZATION
Bone mineralization involves complex physiology.10,11 Osteoblasts are the cells that produce a bone matrix, called osteoid that must be subsequently mineralized by hydroxyapatite crystals. Osteoclasts are the cells that reabsorb bone and return calcium and phosphate to the bloodstream. Mineralization requires normally functioning enzymes, vitamins, and normal circulating serum levels of calcium and phosphate. Bone mineralization is continually being modified for repair and growth and for maintenance of circulating calcium levels.10,11
A subjective assessment of bone mineralization can offer clues to disease processes. Seeing a number of healthy children imaged radiographically for acute processes such as fracture is the best method of training the eye for recognition of poorly mineralized bones. Features to assess include the overall density of the bones, abnormal prominence of the trabeculae that implies demineralization, and thickness of the cortices. Differentiating between osteoporosis, wherein calcium and other minerals have been pulled from the bone and transferred to the vascular circulation, and osteomalacia, a disorder of abnormal bone matrix/osteoid formation, is not always possible. In these cases, the term osteopenia may be used. One example of osteoporosis in the child is seen in the context of immobilization of a limb for the treatment of fracture. When non-weight bearing, osteoclastic activity increases, and calcium content within the affected bones lowers substantially enough that the intramedullary bony trabeculae appear prominent and the subcortical bone becomes more lucent (Fig. 28.3).12,13
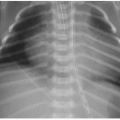
Contrast that appearance with osteomalacia, exemplified by rickets in children. Rickets is a complex disorder of the growth plate and of enchondral ossification, resulting in deficient mineralization and overgrowth of cartilage.11 It is most commonly caused by nutritional vitamin D deficiency.14 Rickets can be recognized radiographically by overall poor mineralization of the skeleton, widening of the growth plates, and irregularity or fraying of the metaphyses. Because these changes are best seen in fast-growing long bones, radiographs of the wrist and knee are most helpful (Fig. 28.4A). Anterior rib metaphyseal widening may be suspected clinically in profoundly affected children by the described “rachitic rosary,” referring to the beadlike palpable nodularity of the chest wall, also apparent radiographically as expansion of the
costochondral junctions (Fig. 28.4B). Typical therapy for rickets is nutritional replacement of vitamin D (Fig. 28.4C).
costochondral junctions (Fig. 28.4B). Typical therapy for rickets is nutritional replacement of vitamin D (Fig. 28.4C).
Formal bone mineralization evaluation by dual x-ray absorptiometry (DEXA) scanning, a subject beyond the scope of this chapter, is indicated for the following conditions: systemic longterm steroids, chronic inflammatory disorders, hypogonadism, prolonged immobilization, osteogenesis imperfecta, idiopathic juvenile osteoporosis, recurrent low-trauma fractures, and apparent osteopenia on radiographs.15,16
SKELETAL ALIGNMENT DISORDERS
Scoliosis
Scoliosis is defined as the presence of at least one abnormal vertebral column curve of greater than 10 degrees in the coronal plane.17, 18 and 19 There are usually accompanying rotations or curvature in other dimensions as well. Scoliosis falls into different categories, depending on its etiology. Approximately 80% to 90% of scoliosis cases are idiopathic, and these cases can develop in infancy, in childhood, or in adolescence.19,20 Less common are scoliosis cases that are congenital (due to vertebral segmentation-fusion anomalies), neuromuscular (due to spinal cord abnormalities such as syrinx or tumor or due to hypotonia conditions such as spinal muscular atrophy), or mesenchymal (due to weakness of passive support mechanisms of the spine, as in Marfan syndrome, skeletal dysplasias, and inflammatory conditions).
Radiography, CT, and MR imaging are all useful in evaluating scoliosis. The primary role in diagnostic imaging is to identify the etiology of the abnormal curvature. Following that, imaging is used to monitor progression of scoliosis and guide orthopedic intervention.18,20 Radiographic assessment of the spine is done with upright views in frontal (PA) and lateral projections. Any observed curve is assessed by measuring the Cobb angle, which is the angle of the extrapolated lines drawn along the steepest endplate at the top and bottom of the curve (Fig. 28.5). Vertebral balance in coronal plane
is measured by drawing a plumb line from C7 straight inferiorly (parallel to the lateral margin of the radiograph). If this line is at least 2 cm lateral to the central sacral vertical line, then coronal imbalance is diagnosed (Fig. 28.5). A similar measurement can be made in the sagittal plane. Additional radiographic assessment on upright spine radiography includes a description of the iliac apophyseal ossification stage using the Risser index, which is done to estimate the stage of skeletal maturity. Absence of apophyseal ossification is Risser grade 0. Apophyseal ossification begins laterally and extends medially, with each quartile along the superior margin of the iliac crest representing Risser grades 1 through 4 (Fig. 28.5). Complete visualization of the ossified apophysis takes approximately 1 year, and fusion of the ossification center to the iliac crest takes an additional 2 years.21 Risser grade 3 or higher in females indicates skeletal maturity, and Risser grade 5 in males indicates maturity.19
is measured by drawing a plumb line from C7 straight inferiorly (parallel to the lateral margin of the radiograph). If this line is at least 2 cm lateral to the central sacral vertical line, then coronal imbalance is diagnosed (Fig. 28.5). A similar measurement can be made in the sagittal plane. Additional radiographic assessment on upright spine radiography includes a description of the iliac apophyseal ossification stage using the Risser index, which is done to estimate the stage of skeletal maturity. Absence of apophyseal ossification is Risser grade 0. Apophyseal ossification begins laterally and extends medially, with each quartile along the superior margin of the iliac crest representing Risser grades 1 through 4 (Fig. 28.5). Complete visualization of the ossified apophysis takes approximately 1 year, and fusion of the ossification center to the iliac crest takes an additional 2 years.21 Risser grade 3 or higher in females indicates skeletal maturity, and Risser grade 5 in males indicates maturity.19
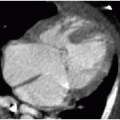
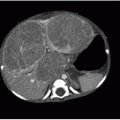
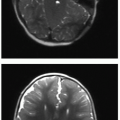
Further evaluation of scoliosis with CT is performed for congenital scoliosis from abnormal bony fusion or segmentation defects or to evaluate a primary osseous lesion (Fig. 28.6). The bony anatomic detail acquired through CT guides surgical planning. The use of MR imaging in the evaluation of presumed idiopathic scoliosis is controversial. Neurologic abnormalities found in patients with presumed idiopathic scoliosis have been reported to be as high at 18%.22 Generally, MR imaging (Fig. 28.7) is pursued in the context of idiopathic scoliosis if any of the following conditions are present: age less than 10 years, signs of neurologic deterioration, rapidly progressive scoliosis, foot deformity, back pain, headache, or unusual radiographic features (discussed below).
Adolescent idiopathic scoliosis has an overall prevalence of 0.5% to 5% and is seen more commonly in females than in males.20 Thoracic right-convex curves are the most common (48%), followed by left-convex thoracolumbar/lumbar curves (40%). Biconvex (S-shaped) curves (12%) are less common (Fig. 28.8).23 Radiographic evidence of a structural cause for scoliosis include the following: curve types commonly associated with neuropathy (left thoracic, double thoracic, short-segment, or long right thoracic curve), severe curvature after skeletal maturity, wide spinal canal, thin pedicle, wide neural foramina, or other features suggestive of a nonosseous lesion leading to bony remodeling.19 The factors that have the greatest effect on the probability of progression of adolescent idiopathic scoliosis are spinal growth velocity and magnitude of the curve at initial presentation.19 The recommended treatment for adolescent idiopathic scoliosis is observation (follow-up at 4- to 12-month intervals) when the Cobb angle is less than 20 degrees, bracing when the Cobb angle is 20 to 45 degrees, and surgery when the Cobb angle is greater than 45 degrees.19




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree