Jean Noel Buy1 and Michel Ghossain2
(1)
Service Radiologie, Hopital Hotel-Dieu, Paris, France
(2)
Department of Radiology, Hotel Dieu de France, Beirut, Lebanon
37.1 Introduction
37.2 Definitions
37.2.1 Pelvic Floor Relaxation
37.2.2 Prolapse
37.3 Clinical Findings
37.3.1 Pelvic Organ Prolapse (POP)
37.3.2 Rectal Prolapse
37.3.4 Incontinence
37.4.1 Active Support: Levator Ani
37.4.2 Passive Support
37.4.3 Sphincters
37.4.4 Urogenital Diaphragm
37.5 Imaging
37.5.1 Indication
37.5.2 MRI
37.6 Conclusion
Abstract
The pelvic floor is a complex system, with passive and active components that provide pelvic support, maintain continence, and coordinate relaxation during urination and defecation [1].
37.1 Introduction
The pelvic floor is a complex system, with passive and active components that provide pelvic support, maintain continence, and coordinate relaxation during urination and defecation [1].
Whereas exact mechanisms of pelvic floor weakness are subject to debate, established risk factors [2–4] include vaginal childbirth, advancing age as pelvic floor weakness progresses with age, and increasing body mass index. Potential risk factors include ethnicity (most common among Caucasian women, followed by Hispanic, Asian, and Afro-Caribbean women), genetics (higher incidence in some families), collagen-related disorders [5], hysterectomy, and repetitive straining (such as chronic constipation or chronic cough) [6].
Pelvic floor dysfunction is an umbrella term for a heterogeneous group of disorders affecting up to 50 % of middle-aged and older women presenting with stress incontinence, pelvic organ prolapse (POP), and defecatory dysfunction (incomplete defecation or fecal incontinence). In addition, over the next 30 years, the population of women over the age of 60 years is expected to increase at a higher rate than the general population, resulting in a projected 45 % increase in the demand for all services related to treating patients with pelvic floor disorders [7].
Most patients with incontinence and minimal pelvic floor weakness can be treated on physical examination and basic urodynamic findings. But around 15 % of the patients are more symptomatic, and by the age of 70 years, an estimated one in ten undergoes pelvic floor surgical repair. Moreover, despite surgeons’ best efforts, symptoms recur in 10–30 % of patients [8]. Although the factors that lead to failure of surgical repair are not well understood, it appears likely that an incomplete physical examination, along with the failure to recognize defects in other compartments that are asymptomatic at the initial evaluation, is contributory for this failure.
Consequently, the treatment of pelvic floor dysfunction is becoming increasingly dependent on accurate preoperative imaging to elucidate the presence and extent of pelvic floor abnormalities.
37.2 Definitions
Pelvic organ prolapse and pelvic floor relaxation are two related and often coexistent but separate pathologic entities, which need to be defined and differentiated.
37.2.1 Pelvic Floor Relaxation
There are two components of pelvic floor relaxation: one anteroposterior with a widening of the puborectal hiatus and one vertical with pelvic floor descent.
37.2.2 Prolapse
Pelvic organ prolapse (POP), also called urogenital prolapse, is downward descent of the pelvic organs that results in a protrusion through the urogenital hiatus.
Colpocele is bulging of this organ in the vagina.
POP is distinct from rectal prolapse that is protrusion of the rectum through the anus as a rectal intussusception.
Different types of POP
Surgeons view the female pelvis as three functional compartments: anterior compartment (bladder and urethra), middle compartment (vagina, cervix, uterus, and adnexa), and posterior compartment (peritoneum and content, rectum and anus).
Prolapse can affect the anterior vaginal wall (anterior colpocele), posterior vaginal wall (posterior colpocele), and uterus or apex of the vagina (apical prolapse), usually in some combination.
Anterior compartment
(a)
Urethrocele or urethral hypermobility is defined as horizontal translation of the urethra away from the normal vertical axis. The angle of the urethral axis is abnormal when it is greater than 30° from the vertical.
(b)
Cystocele results in an anterior colpocele with herniation of the posterior bladder wall through the anterior vaginal wall.
Middle compartment
Apical prolapse is protrusion of the uterus or the vaginal cuff in case of hysterectomy in the vagina or beyond it.
Posterior compartment
Posterior Colpocele (Fig. 37.1)
(a)
Peritoneocele/enterocele/sigmoidocele
These are a herniation of the pelvic peritoneal sac and contents (fat = peritoneocele, small bowel = enterocele/mesenterocele, and less frequently sigmoid = sigmoidocele) beyond the normal confines of the cul-de-sac, through rectovaginal septum, protruding into the posterior vaginal wall.
(b)
Rectocele is herniation of the anterior wall of the rectum above the anal canal through the posterior vaginal wall. Less frequently in context of prior surgery, rectocele can involve the posterior or lateral wall of the rectum.

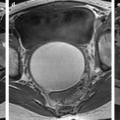
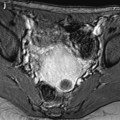
Fig. 37.1
Posterior colpocele in a 78 years old woman with prior hysterectomy. (a) resting midsagittal T2 weighted image and (b) corresponding midsagittal T2 weighted image at maximal straining image show a peritoneocele with peritoneum in rectovaginal space (white arrowhead) bulging to the posterior vaginal wall (black arrowhead). Note the presence of a small rectocele
Rectal prolapse
Rectal prolapse is the protrusion of either the rectal mucosa or the entire wall of the rectum. Partial prolapse involves only the inner lining of the rectum (rectal mucosa) and is usually anterior and protrudes by a few centimeters. Complete prolapse involves all layers of the rectal wall with both the mucosa and muscular layer and is usually circumferential.
37.3 Clinical Findings
37.3.1 Pelvic Organ Prolapse (POP)
37.3.1.1 Physical Examination
Women presenting with symptoms suggestive of POP undergo pelvic examination. Assessment of POP should be done with the patient resting and straining while supine and standing to define the extent of the prolapse and establish the segments of the vagina affected (anterior, posterior, or apical) (Fig. 37.2).




Fig. 37.2
Photographs in lithotomy position. Pelvic organ prolapse might include (a) bladder (cystocele), (b) uterus (hysterocele), (c) peritoneum (peritoneocele), (d) rectum (rectocele)
POP can involve any combination of the following:
Anterior compartment
Urethrocele (also called urethral hypermobility)
In cases where there is loss of fascial support, an increase of abdominal pressure with coughing or sneezing allows the urethrovesical junction descent and rotation of the urethral axis into the horizontal plane. Bladder pressure rises above urethral pressure, resulting in urine leakage. Thus, patients with urethral hypermobility can have urinary incontinence, and urine leakage may be seen when the patient is asked to perform a Valsalva maneuver. The diagnosis of urethral hypermobility is made at physical examination and cystourethroscopy. Direct observation of urethral mobility is made by placing a Q-tip at the urethrovesical junction and asking the patient to strain.
Cystocele: anterior colpocele
As the bladder neck and proximal urethra are mobile, descent of the bladder neck during strain may result in clockwise rotational descent of the bladder neck and proximal urethra and can cause kinking of the proximal urethra that may mask stress urinary incontinence and may be a potential cause of urinary retention leading to urinary stasis and infection.
Other signs include a vaginal mass with symptoms of heaviness and bulging of tissues in the vagina that are exacerbated by physical exertion or standing for prolonged periods of time and back pain if the bladder prolapse is severe enough as the muscular pelvic floor may entrap the ureters and create ureteral obstruction (hydronephrosis).
Middle compartment
Apical prolapse entails either the uterus or post-hysterectomy vaginal cuff
In severe cases of complete eversion, cervical and uterine prolapses (procidentia) are seen as a bulging mass outside the external genitalia as the vaginal walls form a sac containing the prolapsing organs. If this prolapse is long standing, the mucosa can become thick. Patients with vaginal eversion have difficulty walking or sitting as well as pelvic pressure and protrusion of tissue through the vagina.
Posterior compartment: posterior colpocele
Posterior vaginal wall prolapse (or a posterior colpocele) involves the rectum, peritoneum with or without bowel, or both.
Peritoneocele/enterocele/sigmoidocele
On physical examination, enlargement of the enterocele may produce a bulge at the introitus and the presence of a bulge in the superoposterior vaginal wall. The examiner places one finger in the vagina and another one in the rectum. The presence of small bowel in the rectovaginal space is suggested by loops of small bowel palpated between an examiner’s fingers or by observation of peristalsis behind the posterior vaginal wall. Peristalsis of small bowel may also be appreciated if the vaginal wall is thin. The incidence of enterocele has markedly increased as a result of the widespread performance of both hysterectomy and cystourethropexy because both of these procedures open up the posterior cul-de-sac.
Patients may have a dragging sensation in the pelvis as well as pelvic pressure. Stretching of the mesentery with straining can cause pain in the lower abdomen or back. An enterocele may result in compression of the distal part of the anorectum and finally in incomplete evacuation due to outlet obstruction.
Peritoneocele/enterocele may not always be detected clinically or can also be confused with high rectoceles. Exceptionally peritoneocele can occur between the bladder and the vagina through the vesicovaginal septum (only in the context of hysterectomy).
Rectocele
A rectocele is diagnosed when a coincident anterior and inferior rectal wall bulge occurs during evacuation. Most are not apparent at rest. Rectoceles are common in women because the rectovaginal septum is relatively weak, and rectoceles are likely to be a normal variant [9] but only become clinically significant when symptoms develop. Patients may present with obstructed defecation or incomplete evacuation as the association between a large rectocele and difficult evacuation is well recognized. Many need to assist defecation by applying digital pressure to the perineal body or posterior vaginal wall. Asymptomatic rectoceles are present in up to 80 % of patients with multicompartment disease.
Other symptoms include a bulge along the superoposterior wall of vagina, perineal fullness, dyspareunia, and low back pain.
Very uncommonly, posterolateral herniation of the rectum may result from levator ani damage during childbirth or prior to surgery in the rectal region.
37.3.1.2 Clinical Classification
Although several prolapse grading systems exist, the only system with international acceptance is the pelvic organ prolapse quantification (POP-Q) system [10].
The amount of prolapse is systematically defined during a pelvic examination by measuring anterior, posterior, and apical segments of the vaginal wall in centimeters relative to a fixed anatomical structure (the vaginal hymen). Six points are measured with reference to the plane of the hymen. Points above or proximal to the hymen are described by the distance from the hymenal plane in centimeters preceded by a minus sign, while points below or distal to the hymen are described by the distance in centimeters preceded by a positive sign. Three other measurements include total vaginal length, genital hiatus, and perineal body, for a total of nine points, which can be documented on the 3 × 3 grid (Fig. 37.3). Enterocele is not a part of the POP-Q system and should be noted by the examiner.


Fig. 37.3
Graphic of the different points and measurements of POP Q system and the 3×3 grid. Anterior Vaginal Wall: Point Aa corresponds approximately to the urethrovesical junction. Point Ba: Represents the most distal position of any part of the upper anterior vaginal wall from the vaginal cuff or anterior vaginal fornix to point Aa. Posterior Vaginal Wall: Posterior vaginal wall points are measured with the woman straining after single speculum blade is turned to retract the anterior vaginal wall. Point Ap: Located in the midline of the posterior vaginal wall, 3 cm proximal to the hymen. Point Bp: The most distal position of any part of the upper posterior vaginal wall from the vaginal cuff or posterior vaginal fornix to point Ap. Superior Vagina: Points located on the superior vagina represent the most proximal location of the normally positioned pelvic organs. These points are assessed with the woman straining with an assembled speculum in place. Point C: The most distal (most dependent) edge of the cervix or the leading edge of the vaginal cuff after hysterectomy. Point D: Marks the location of the posterior fornix in a woman who still has a cervix. Additional Measurements: Total Vaginal Length (TVL): With an assembled speculum in place and point C or D reduced completely to its normal position, the total vaginal length is measured as the greatest depth of the vagina in centimeters. Genital Hiatus (GH): Measured from the middle of the external urethral meatus to the posterior midline hymen while the patient is straining. Perineal Body (PB): Measured from the posterior margin of the GH to the midanal opening
Prolapse is quantified by stages, which are assigned according to the most severe portion of the prolapse when the maximum protrusion has been demonstrated (Table 37.1).
Table 37.1
Quantification of stages of prolapse
Stage | Definition |
|---|---|
Stage 0 | Points Aa, Ap, Ba, and Bp are assigned value of −3 cm. Value for point D or C is less or equal to TVL (cm) −2 cm and has a negative sign. This stage represents no prolapse |
Stage I | The most distal portion of the prolapse is more than 1 cm above the level of the hymen |
Stage II | The most distal portion of the prolapse is 1 cm or less above the level of the hymen or within one centimeter below the hymen |
Stage III | The most distal portion of the prolapse is more than 1 cm below the hymen, but no further than 2 cm less than the total vaginal length in centimeters |
Stage IV | Almost complete eversion of the total length of the vagina is present. The protrusion minimally extends beyond hymen further than TVL-2 cm |
37.3.2 Rectal Prolapse
It is important to understand that POP is distinct from rectal prolapse, in which the rectum protrudes through the anus as a rectal intussusception.
Although mucosa alone may prolapse (anterior mucosal prolapse), invagination or intussusception is defined as a full-thickness rectal wall prolapse involving both the mucosa and muscular layer. Both can be associated with a rectocele during straining. Infolding of the rectal wall may be intrarectal, intra-anal, or complete rectal prolapse. Extra-anal invagination is currently clinically termed rectal prolapse [11]. This causes a mechanical obstruction to the passage of stool. Similar to a rectocele, small degrees of rectal intussusception can also be seen in asymptomatic individuals (50 % in volunteers [9]). Rectoanal or extra-anal intussusceptions can be associated with rectal ulcer.
37.3.3 Spastic Pelvic Floor Syndrome
Spastic pelvic floor syndrome (also known as anismus, pelvic floor dyssynergy, or paradoxical puborectalis contraction) is a functional abnormality that affects some constipated patients who experience evacuation failure associated with involuntary, inappropriate, and paradoxical contraction of striated pelvic floor musculature (mainly the puborectalis muscle) [12]. The etiology of this condition is unclear and can include both abnormal muscle activity and psychological or cognitive factors [1]. Increased pressure at rest and during defecation is shown with anorectal manometry, while pathological signals are evident at electromyography.
37.3.4 Incontinence
37.3.4.1 Urinary Incontinence
Urinary incontinence has been defined as “a condition in which involuntary loss of urine is a social or a hygienic problem and is objectively demonstrable” [13].
Urinary incontinence in women is divided into stress urinary incontinence, urge urinary incontinence, and overflow urinary incontinence. Stress urinary incontinence is defined as uncontrolled urine leakage during stress or activity such as sneezing, coughing, or exercise. Two types of stress urinary incontinence are identified: genuine stress incontinence, caused by hypermobility of the bladder neck, and the less frequent intrinsic sphincter deficiency, caused by a sphincter defect. Urge incontinence and overflow urinary incontinence are related to bladder abnormalities. In urge incontinence, there is detrusor overactivity due to damage to the nervous system supplying the urinary bladder, such as in multiple sclerosis, stroke, or pelvic injury, and a large amount of urine leaks when the patient experiences a sudden urge to urinate. In overflow incontinence, a small amount of urine leaks when the urinary bladder is overdistended because of weakness of the bladder muscles in a neurogenic bladder or in chronic bladder outlet obstruction.
37.3.4.2 Anal Incontinence
Anal incontinence is common, especially in women, with a prevalence increasing with age, and has a considerable economic impact [14]. Patients can present with either fecal leakage suggesting an internal sphincter abnormality or with urge incontinence, indicating external sphincter damage. The most common cause of incontinence is vaginal delivery that causes direct sphincter laceration or indirect damage to sphincter innervation. Other causes include iatrogenic damage (as a complication of anal surgery) or neuropathy.
37.4 Anatomy of Pelvic Floor Structures and Physiopathology
The pelvic floor is a complex, integrated, multilayered anatomic and functional unit that provides active and passive support. Endopelvic fascia and ligaments to the bony pelvis provide passive support, while the muscles of the pelvic floor, mainly the levator ani, provide active support.
Support of the pelvic structures depends on the interplay between the fascia and ligaments and the levator ani muscle. Disruption with tears or dysfunction (weakness) of both or one of the structures may be compensated for by the others but will predispose to eventual pelvic floor disorders leading to various degrees and combinations of pelvic organ prolapse and pelvic floor relaxation.
37.4.1 Active Support: Levator Ani
37.4.1.1 Anatomy
In many mammal quadruped species, the levator ani is composed of different muscles which are distinct, the ones of the cloacae sphincter and the ones of the caudo-pelvic muscle. But in primates, the levator ani is a broad muscular sheet which is subdivided into named portions according to their attachments and the pelvic viscera to which they are related. These parts are often referred to as separate muscles, but it must be kept in mind that the boundaries between each part cannot be easily distinguished. Considering the Terminologia Anatomica (international anatomical terminology), the levator ani complex comprises five distinct origin-insertion pairs or subdivisions [15], each with its own unique mechanical effect. The puboanalis, puboperinealis, and pubovaginalis muscles form a single mass in some regions and are separately classified because of their different insertion points. They are, therefore, displayed together as a single structure for visual clarity referred to the term pubovisceralis (pubococcygeus). The two other parts are the puborectalis and iliococcygeus muscles (Fig. 37.4).


Fig. 37.4
View from above of the pelvic floor. The three major subdivisions of the right part of the levator ani are contoured: the pubovisceralis (pink), the puborectalis (brown), and the iliococcygeus (purple). Urethra cut (black arrow), vagina cut (black cross), rectum cut (white star), arcus tendineus of levator ani (arrow heads), ischiococcygeus ligament (black star), red mark (right ischial spine), blue mark (left ischial spine), green mark (sacrococcygeus junction), yellow mark (tip of the coccyx)
To simplify, two perpendicular components can be described [16]: an inferomedial part (composed of the pubovisceralis and the puborectalis) which is central, vertical, thick, and has sphincter function and the superoposterolateral part (composed of the iliococcygeus) which is lateral, horizontal, thin, and has a function of elevating.
The inferomedial part of the levator ani is attached to the back of the body of the pubis and passes back almost vertically. It can be divided in two parts: the pubovisceralis and the puborectalis. The pubovisceralis lies medial to the puborectalis, arises high on the pubis near the superior pubic ramus where it forms a single mass, and is then divided into separate parts according to the pelvic viscera to which they relate: the pubovaginalis, puboperinealis, and puboanalis muscles which are separately classified because of their different insertion points, the vagina, perineal body, and anus, respectively. The puborectalis arises near the inferior pubic ramus and passes, as a thick muscular sling, behind the rectum around the anorectal junction just cephalad to the anal sphincter.
The superoposterolateral part (iliococcygeus part) is a wide, thin, and horizontal sheetlike structure attached to the inner surface of the ischial spine below and anterior to the attachment of the ischiococcygeus and to the obturator fascia as far forward as the obturator canal. The most posterior fibers are attached to the tip of the sacrum and coccyx, but most join with fibers from the opposite side to form the raphe (anococcygeal ligament).
The innervations of the levator ani consist of fibers originating mainly in the second, third, and fourth sacral spinal segments to reach the muscle from below and above in a variety of routes.
The levator ani is supplied by branches of the inferior gluteal artery, the inferior vesical artery, and the pudendal artery.
Unlike other skeletal muscles in the body, in healthy women, the LA remains in constant tone even during rest. It is well recognized that the levator ani must relax appropriately to permit expulsion of urine and particularly feces. This striated muscle has slow-twitch (type I) muscle fibers [17] that continuously contract at rest and provide tone to the pelvic floor, against the stress that comes from gravity and intra-abdominal pressure. Its activity automatically adjusts to variations in posture and abdominal pressure to provide upward support to the pelvic viscera. Its action minimizes the load on connective tissues that attach the organs to the pelvis.
Tonic contraction closes the urogenital hiatus and provides a supportive platform during normal activity and standing. At rest, by forming a U-shaped sling between the pubis and the anus, the puborectalis particularly acts as an anterior sling keeping the rectum, vagina, and urethra elevated and closed by pressing them anteriorly toward the pubic symphysis, resulting in an acute angle between the bladder neck and the urethra as between the anus and the rectum.
37.4.1.2 MRI
The subdivisions of the levator ani muscle are clearly seen in MRI scans on T2-weighted MR images, each with distinct morphology and characteristic features [18].
The inferomedial part (pubovisceralis and puborectalis) (Figs. 37.5, 37.6, and 37.7)


Fig. 37.5
Axial T2-weighted scan of a 22-year-old nullipara volunteer showing subdivisions of the levator ani from bottom to top. (a) axial image shows the puboperinealis (pp) entering the perineal body (pb). (b) axial image shows the anterior retropubic part of the pubovisceralis (PV) and distal insertions with attachment to the vaginal wall (the pubovaginalis (pv)) and with attachment to the anal canal wall (the puboanalis (pa)). (c) axial image shows the puborectalis (PR) appearing as a sling dorsal to the rectum. (d–f) axial images show the iliococcygeus (IC) arising from the arcus tendineus levator ani (arrow head) and passing around the rectum above the fibers of the puborectalis to form the anococcygeal raphe (black arrow). Note that the iliococcygeus is up to the ischial spine (white arrow) behind the ischiococcygeus (Ic)


Fig. 37.6
Coronal T2-weighted scan of a 22-year-old nullipara volunteer showing subdivisions of the levator ani from back to forth. (a, b) coronal images show well the two perpendicular components of the levator ani with its inferomedial part composed of the pubovisceralis and the puborectalis (PV + PR) and its superoposterolateral part composed of the iliococcygeus (IC). (c, d) coronal images show the iliococcygeus (IC) with its winglike configuration arising from the arcus tendineus levator ani (arrow head) up to the ischial spine (white arrow)

Fig. 37.7
Sagittal T2-weighted scan of a 22-year-old nullipara volunteer showing subdivisions of the levator ani from right to left. (a, c) sagittal images show the shelflike orientation of the iliococcygeus (IC) which is dorsal and above the pubovisceralis and puborectalis (PV + PR). (b) median sagittal image shows the puborectalis that passes dorsal to the rectum with decussating fibers forming a “bump” (star)
The axial plane provides a clear view of the pubovisceralis and its subdivisions. Near the pubic origin, the pubovisceralis subdivisions cannot be distinguished. However, the different insertion points of the pubovaginalis, puboperinealis, and puboanalis muscles can be seen. The pubovaginalis attachment is identified as medial fusion of the muscle with the vaginal wall. The puboperinealis muscle can be seen entering into the perineal body. The puboanalis portion can be seen passing into the intersphincteric space.
The puborectalis is a subdivision that can be distinguished from the three elements of the pubovisceralis. It originates lateral to the pubovisceralis and appears as a sling dorsal to the rectum. The aspect of the right puborectalis may appear slightly thinner than the left, a finding that is caused by chemical shift artifact in most cases.
The coronal view is perpendicular to the fiber direction of the puborectalis and pubovisceralis and shows them as “clusters” of muscle on either side of the vagina because their fibers are contiguous and without visible separation; the two together are seen as a single body of muscle lateral to the vagina. Similarly, the subdivisions composing the pubovisceralis cannot be separated in the coronal MRI cross sections.
The sagittal plane offers the distinct advantage of being parallel to the pubovisceralis and puborectalis fiber directions, allowing the fibers of the puborectalis to be seen as they pass dorsal to the rectum forming a “bump” that is consistently visible.
The coronal plane is optimal for viewing the iliococcygeus in the dorsal parts of the pelvis. This winglike configuration is visible as it arises from its lateral attachments to the arcus tendineus levator ani over the obturator internus muscle. On coronal images, the iliococcygeal muscle should be intact and upwardly convex. As women age, some thinning of the levator ani muscle occurs normally; however, no tears should be identified. In axial images, it can be seen passing around the rectum above the fibers of the puborectal muscle. The sagittal images show the shelf like orientation of the iliococcygeus muscle, which has been referred, with the addition of the anococcygeal raphe, as the “levator plate” by various authors.
37.4.1.3 Pathology
The injury to the levator ani plays a significant role in prolapse [19, 20], and different studies add to a growing body of data concerning connective-tissue abnormalities [21] and smooth muscle [22, 23] in the development of prolapse. For instance, it is plausible that failure in one element (levator ani muscle) could lead to increased demands on other components (connective tissue and smooth muscle) which may eventually fail as well. The fact that 30 % of the women with prolapse had no evidence of a muscle defect on MRI supports the fact that the disease process involves other factors as well. This fact does not diminish the importance of levator ani injury but is a reminder that multiple aspects of the system underlie failure. Decline of normal levator ani tone – by denervation or direct muscle trauma – results in an open urogenital hiatus, weakening of the horizontal orientation of the iliococcygeus, and a bowl-like configuration [24].
Each component of the levator ani muscle has a unique origin-insertion pair and, thus, a unique line of action. An injury to each individual subdivision of the levator ani would be expected to have a specific functional effect resulting in a unique deficit. But there are no clear-cut boundaries between normal and abnormal morphologic condition of the pelvic floor considering the morphologic variants of the levator ani muscle [19, 25]. For example, the absence of a visible insertion of the LA inside the pubic bone on both sides in 10 % of all women can be a surprising result, because all anatomy books describe this connection. If some of anatomic variants that may favor prolapse after vaginal birth may be questioned, for now, findings on MRI can be regarded as pathological only if the corresponding clinical symptoms are present.
In addition to direct muscle trauma, neuropathic injury of the levator ani muscles can result from vaginal delivery [26]. Chronic straining to achieve defecation has also been associated with pelvic muscle denervation [27]. The excess straining and associated perineal descent are thought to cause stretch injury to nerve and result in neuropathy.
37.4.2 Passive Support
37.4.2.1 Bone
The bony pelvis and lumbar osseous system plays a role in the orientation of the forces to the pelvic structures. Variations in orientation and shape of the bony pelvis have been associated with development of pelvic organ prolapse. Specifically, a loss of lumbar lordosis and a pelvic inlet that is less vertically oriented is more usual in women who develop genital prolapse than in those who do not [28, 29]. A less vertical orientation of the pelvic inlet is thought to result in alteration of intra-abdominal vector forces that are usually directed anteriorly to the pubic symphysis such that a greater proportion is directed toward the pelvic viscera and their connective-tissue and muscular supports.
37.4.2.2 Fascia
Anatomy
The endopelvic fascia is the connective-tissue network that envelops all organs of the pelvis and connects them loosely to the supportive musculature and bones of the pelvis. The endopelvic fascia and the ligaments formed from fascia are not seen on MRI.
Anteriorly, the endopelvic fascia forms a supportive layer called the pubocervical fascia between the pubis, the urinary bladder, and the anterior vaginal wall. It attaches to the arcus tendineus fasciae pelvis anterolaterally and to the cervix posteriorly and attaches the lateral part of the vagina to the pelvic sidewalls.
In the middle compartment, the endopelvic fascia also attaches the uterus-cervix and vagina to the pelvic sidewall via the elastic condensations known as the parametrium and paracolpium, respectively. The parametrium is made up of the cardinal and uterosacral ligaments (which suspend the uterus and upper vagina from the presacral fascia) and provides support to the body of the uterus. The uterosacral ligaments suspend the cervix and upper vagina from the presacral fascia. This attachment pulls these structures superiorly and posteriorly over the levator ani toward the sacrum. This mechanism is protective because the vagina moves into the hollow of the sacrum and forms a horizontal supporting shelf when abdominal pressure increases. The paracolpium stretches the vagina transversely between the bladder and the rectum. Vaginal support in the pelvis has been described by DeLancey [30] as having three levels of support. The cephalic 2–3-cm portion of the vagina, described as level 1, is suspended from the pelvic sidewall by the parametrium and paracolpium, which are condensations of the endopelvic fascia. Level 3 is described as the level that starts at the hymen ring and extends 2–3 cm cephalad to it, and level 2 is between levels 1 and 3. Level 2 of the vagina is attached to the arcus tendineus, although level 3 is directly attached to its surrounding structures: anteriorly to the urethra, posteriorly to the perineal body, and laterally with the levator ani muscles, rather than being attached to or suspended from the pelvic walls.
Posteriorly the endopelvic fascia forms a supportive layer, the rectovaginal fascia, between the posterior vaginal wall and the rectum, which attaches the posterior part of the vagina to perineal body and prevents the rectum from protruding forward and the bowel from herniating inferiorly. One of the muscles of the levator ani, the iliococcygeus muscle, is a horizontally oriented sheet of muscle that, together with the rectovaginal fascia, forms a diaphragm that provides support to the pelvic organs, especially those in the posterior compartment.
Two dense aggregations of obturator and levator ani fascia, the arcus tendineus fasciae pelvis and arcus tendineus levator ani, provide important passive lateral support. The arcus tendineus fasciae pelvis provides lateral anchoring for the anterior vaginal wall where it underlies and supports the urethra, while the arcus tendineus levator ani provides anchoring for the levator ani muscles. While the arcus tendineus levator ani can be identified on MRI images as the origin of the iliococcygeus at the internal obturator fascia, the arcus tendineus fasciae pelvis cannot be visualized on MRI.
Pathology
Disruption or stretching of these connective-tissue attachments happens during vaginal delivery or hysterectomy, with chronic straining, or with normal aging [8].
In the anterior compartment, defects in the pubocervical fascia with loss of support to the urethra and bladder can allow urethral hypermobility and ultimately a cystocele. Four types of defects can occur in the pubocervical fascia: lateral or paravaginal defects when the pubocervical fascia detaches from the arcus tendineus fasciae pelvis, transverse defects when the pubocervical fascia detaches from its central attachment at the pericervical ring of fibrous tissue, central defects when the pubocervical fascia is disrupted in the midline under the bladder base, and distal defects when the fascia detaches from the urogenital diaphragm. The most common type of defect is the paravaginal defect, which may be unilateral or bilateral and is often seen in patients with urethral hypermobility. More important stretching or tearing of the pubocervical fascia and levator ani muscle allows the posterior aspect of the bladder to bulge into the anterior vaginal wall.
In the middle compartment, abnormalities of the pubocervical fascia, parametrium, paracolpium, or uterosacral ligaments allow mobility and descent of the uterus, cervix, or vaginal cuff. In patients who have undergone a hysterectomy, prolapse of the vaginal apex can arise because of weakness of the paracolpium, resulting in apical prolapse.
In the posterior compartment, prolapse of peritoneal contents is due to deficiency of the supporting ligaments, the rectovaginal fascia and iliococcygeus muscle, resulting in widening of the rectovaginal space. There are two types of enterocele: the one with a mechanism of traction (follows vaginal/uterine prolapse) and the one with a mechanism of pulsion (occurs with chronic pressure on vaginal vault). In normal individuals, the rectovaginal space caudal to the upper third of the vagina is closely apposed [31]. Widening of this space will allow inferior herniation of the peritoneal fat, small bowel, sigmoid colon, and fluid into the pouch of Douglas. Prior hysterectomy may cause disruption of the rectovaginal fascia and is a risk factor for peritoneocele as a vaginal vault descent causes a traction effect on the cul-de-sac, displacing it inferiorly, and creates a wider potential space for the peritoneal cul-de-sac to descend through or can cause a traction effect on it [1], resulting in a peritoneocele.
A rectocele results from defects in the prerectal and pararectal fascia as well as the rectovaginal septum.
37.4.3 Sphincters
The female urethra is approximately 4.5 cm long, with two-thirds of the urethra above the levator ani. The urethral sphincter is composed of involuntary inner smooth muscle that is continuous with the bladder as well as the voluntary external sphincter, which is composed of striated muscle. The amount of striated muscle decreases with aging, and this may play a role in incontinence. The urethra can be appreciated on MRI (Fig. 37.8), but its different layers and its supporting ligaments are readily visible at high-resolution MR imaging with an endovaginal or endourethral coil on T2-weighted images [1, 32].


Fig. 37.8
Normal urethra of a 24-year-old nullipara volunteer. (a) sagittal T2-weighted image shows the normal position of the urethra (arrow heads). (b) coronal T2-weighted image shows the relationships of the urethra with the levator ani (LA). (c) axial T2-weighted image shows the midportion of the urethra well delineated by the outer striated muscle layer which appears hypointense
The anorectal junction is defined as the point of angulation between rectum and anal canal, corresponding to the posterior impression of the puborectal muscle (Fig. 37.9). In healthy patients, the anorectal angle closes during squeezing, and the anorectal junction can rise 1–2 cm from the rest position. During straining and defecation, the anorectal angle becomes more obtuse, the anal canal opens and shortens, and the rectum is rapidly evacuated. Finally, the anal canal closes, and the anorectal junction and anorectal angle return to their pre-evacuation positions.


Fig. 37.9
Normal anorectal junction of a 24-year-old nullipara volunteer. Sagittal T2-weighted image shows the anorectal junction at the point of the angulation of the rectum and the anal canal. Note the posterior impression of the puborectal muscle (star)
The anal sphincters form two cylindrical layers, between which lies the longitudinal muscle. The internal sphincter forms the innermost muscular layer and is the terminal condensation of the circular rectal smooth muscle. The internal sphincter extends from the anorectal junction to approximately 1 cm below the dentate line. It is composed of smooth muscle fibers with autonomous innervation from sympathetic presacral nerves. The longitudinal muscle is the continuation of the longitudinal muscle of the rectal wall [1]. The deep part of the external anal sphincter is fused with or intimately related to the puborectalis muscle. Anteriorly, it is closely related to the superficial transverse muscle of the perineum and the perineal body. Posteriorly, the muscle is continuous with the anococcygeal raphe. The anal sphincter muscles are well visualized on MRI (Fig. 37.10), but their study is usually not performed during a dynamic study of the pelvis. It can be performed with an endoanal coil after the dynamic evaluation if desired or is usually a separate study [1].


Fig. 37.10
Normal canal anal sphincters of a 22-year-old nullipara volunteer. (a) axial, (b) paramedian sagittal, and (c) coronal T2-weighted images show the external sphincter (red line) wrapped behind the internal sphincter (star)
37.4.4 Urogenital Diaphragm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree