Radiologic Evaluation of Musculoskeletal Infections
Musculoskeletal Infections
Infections of the musculoskeletal system can be subdivided into three categories: (a) those involving bones (osteomyelitis); (b) those involving joints (infectious arthritis); and (c) those involving soft tissues (cellulitis). Because of the complexity of the vertebrae and their soft-tissue structures, infectious processes of the spine are considered under a separate heading.
Osteomyelitis
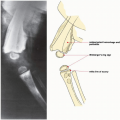
Three basic mechanisms allow an infectious organism—whether bacterium, virus, mycoplasma, rickettsia, or fungus—to reach the bone: (a) hematogenous spread via the bloodstream from a remote site of infection, such as the skin, tonsils, gallbladder, or urinary tract; (b) spread from a contiguous source of infection, such as from the soft tissues, teeth, or sinuses; and (c) direct implantation, such as through a puncture or missile wound or an operative procedure (Fig. 24.1).
Hematogenous spread is common in children, and the usual focus of infection develops in the metaphysis. The metaphyseal location of infection in children is related to an osseous-vascular anatomy that differs in the infant, child, and adult (Fig. 24.2). In the child (ages 1 to 16 years), there is separation of the blood supply to the metaphysis and epiphysis, each having its own source. Moreover, the arteries and capillaries of the metaphysis turn sharply without penetrating the open growth plate; in the region where capillaries become venules, the rate of blood flow is sluggish. Also contributing to the greater incidence of metaphyseal osteomyelitis in children is secondary thrombosis of end arteries with bacteria during transient bacteremia. In the infant (up to 1 year), however, osteomyelitis may sometimes have its focus in the epiphysis because some metaphyseal vessels may penetrate the growth plate and reach the epiphysis (see Fig. 24.2). With obliteration of the growth plate in the adult, there is vascular continuity between the shaft and the articular ends of the bone; hence, the focus of osteomyelitis can develop in any part of a bone.
Contiguous spread and direct implantation are more common in adults. The sites of bone infection via either of these routes are directly related to the focus of soft-tissue infection or the location of the wound.
Infectious Arthritis
An infectious agent may enter the joint by the same basic routes as in osteomyelitis: by direct invasion of the synovial membrane, either secondary to a penetrating wound or after a joint-replacement procedure; from an infection of the adjacent soft tissues; or indirectly via a blood-borne infection. Infectious arthritis may also occur secondary to a focus of osteomyelitis in the adjacent bone (Fig. 24.3).
Cellulitis
Soft-tissue infections most commonly result from a break in the skin leading to direct introduction of an infectious agent. Some patients, such as those with diabetes, are particularly prone to cellulitis caused by a combination of factors, including skin breakdown and local ischemia.
Infections of the Spine
Infections in the spine may be located in a vertebral body, an intervertebral disk, the paravertebral soft tissues, or the epidural compartment; very rarely, an infection may involve the contents of the spinal canal or the spinal cord. The mechanisms of infection are the same as those of osteomyelitis and infectious arthritis. An intervertebral disk infection, for example, may result from a puncture of the canal or of the disk itself during a procedure, as well as from a penetrating injury. It can also spread from a contiguous source of infection such as a paraspinal abscess. Most common, however, is hematogenous spread after surgical procedures such as laminectomy or spinal fusion, or during generalized bacteremia or sepsis (Fig. 24.4). Regardless of the primary location of the infectious process, Staphylococcus aureus is responsible for more than 90% of all infections of the spine.
Radiologic Evaluation of Infections
The imaging modalities used to evaluate infections of the musculoskeletal system include the following:
Conventional radiography
Computed tomography (CT)
Arthrography
Myelography and diskography
Fistulography (sinogram)
Arteriography
Radionuclide imaging (scintigraphy, bone scan)
Ultrasound (US)
Magnetic resonance imaging (MRI)
Percutaneous aspiration and biopsy (fluoroscopy-guided, CT-guided, or US-guided)
Conventional Radiography and Arthrography
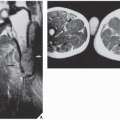
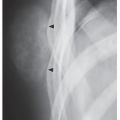
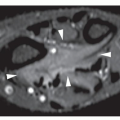
In most instances, radiography is sufficient to demonstrate the pertinent features of a bone or joint infection (Fig. 24.5; see also Figs. 4.50 and 4.51). Magnification radiography used to be helpful in delineating subtle changes representing cortical destruction or periosteal new bone formation (Fig. 24.6), but currently with the advantage of digital radiography and newest technology of PACS (Picture Archive and Communication System) allowing filmless high-resolution image-display format (see Chapter 12), this technique is no longer in practical use. Conventional tomography using multidirectional motion (trispiral tomography) in the past was effective in demonstrating sequestra or subtle sinus tracts in the bone (Fig. 24.7), but at present has been almost completely replaced by CT, which plays a determining role in demonstrating the extent of infection in bones and soft tissues and at times may be very helpful in making a specific diagnosis (Fig. 24.8). Arthrography has rather limited application in the diagnosis of joint infections (see Fig. 25.17B).
Radionuclide Imaging
Scintigraphy has a very prominent role in diagnosing bone and soft-tissue infections. In suspected osteomyelitis, radionuclide bone scan using technetium-99m-labeled (99mTc) phosphonates is routinely used, because there is an accumulation of tracer in the infected areas. A three- or four-phase technique is particularly useful for distinguishing infected joint tissues from infected periarticular soft tissues if radiography is not diagnostic. With cellulitis, diffuse increased uptake is present in the first two phases, but there is no significant increase in uptake in the bone in the third and fourth delayed phases. Conversely, osteomyelitis causes focally increased uptake in all four phases (Fig. 24.9). In addition, the three-phase bone scan can accurately diagnose osteomyelitis within 3 days of the development of symptoms, much earlier than can be seen with conventional radiography. The three-phase bone scan can also be useful in diagnosing septic arthritis in situ or with extension into the adjacent bone.
Once the bone sustains an injury, such as surgery, fracture, or neuropathic osteoarthropathy, that causes increased bone turnover, routine scintigraphy with technetium-labeled phosphonate becomes less specific for infection. However, radionuclide studies using gallium (a ferric analog) and indium are more specific in these instances. There is still no general agreement on the exact mechanism of gallium localization in infected tissues. After intravenous injections of gallium, more than 99% is bound to various plasma proteins, including transferrin, haptoglobin, lactoferrin, albumin, and ferritin. At least five mechanisms of gallium transfer from the plasma into inflammatory exudates and cells have been suggested. These include direct leukocyte uptake, direct bacteria uptake, the protein-bound tissue uptake, increased vascularity, and increased bone turnover. Because gallium binds to the iron-binding molecule transferrin, the mechanism of gallium uptake in infectious processes is best explained by hyperemia and elevated permeability that increase delivery of the protein-bound tracer transferrin into the area of inflammation. Cells associated with the inflammatory response, particularly polymorphonuclear white cells in which lactoferrin is carried within intracytoplasmic granules, deposit iron-binding proteins extracellularly at the site of inflammation, serving to combat the infection by sequestering needed iron from bacteria. Lactoferrin, which has a high binding affinity for iron, takes the gallium away from the transferrin.
Gallium can also be used to assess the patient’s response to therapy. Particularly in osteomyelitis, gallium concentrations enhance the specificity of an abnormal bone scan, and decreased gallium uptake closely follows a good response to therapy.
The other tracer used in infections is indium. Because indiumlabeled white blood cells are usually not incorporated into areas of increased bone turnover, scintigraphy with indium-111 (111In) oxine-labeled leukocytes is used as a sensitive and specific test in the general diagnosis of infection of the musculoskeletal system, and in specific instances when infection complicates previous fracture or surgery. Like other imaging procedures in nuclear medicine, this test monitors the internal distribution of a tracer agent to provide diagnostic information. The inherent ability of white blood cells to accumulate at sites of inflammation makes their use in this test particularly effective in the diagnosis of infections. Merkel reported the sensitivity of indium scintigraphy in detecting infections to be 83%, with a specificity of 94% and an accuracy of 88%.
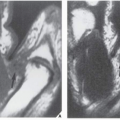
 FIGURE 24.7 Tomography of active osteomyelitis. (A) Radiograph of the left femur shows thickening of the cortex, reactive sclerosis, and foci of destruction in the medullary cavity. Faint calcifications in the soft tissue (arrow) suggest the presence of a fistula. (B) Conventional tomogram enhanced by magnification clearly demonstrates a sequestrum (open arrows) and a sinus tract in the cortex (long arrow), the characteristic features of active osteomyelitis.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|