13 The Adnexa Ovarian cancer accounts for 25 % of all malignancies of the female genital tract. In terms of the absolute number of deaths, it ranks second among gynecologic cancers following breast cancer.1 Early ovarian cancer is often asymptomatic, and most women have advanced disease at presentation.1 A reliable diagnostic test is needed, especially to detect ovarian cancer at an early stage. Transvaginal ultrasound (TVUS) is now the primary imaging modality;2,3 MRI is typically used as a supplementary tool for further characterization of a known ovarian mass. The vast majority of ovarian tumors operated on because of suspected malignancy turn out to be benign cysts. Gynecologists routinely use TVUS to supplement the pelvic examination. Because of the direct access, TVUS can be performed at high frequency and yields high-resolution images.3 MRI is a useful secondary imaging tool in women with complex or solid ovarian masses and indeterminate ultrasound findings.4,5 Tissue characterization based on the analysis of MR signal intensities on different pulse sequences enables reliable differentiation of serous, mucinous, hemorrhagic, fatty, and other solid pelvic masses. Moreover, the known high soft-tissue contrast and multiplanar capability of MRI allow better definition of the spatial relationship of a pelvic mass to other structures in the narrow confines of the true pelvis and to the pelvic sidewall. By thus improving preoperative characterization of ovarian masses, MRI can help reduce overtreatment. Ovarian tumors that are very likely benign based on their MRI appearance can be managed by pelvic laparoscopy without compromising therapeutic outcome, reserving laparotomy for women with suspicious ovarian masses.6 Although expensive, MRI can therefore help contain health-care expenditure when its use is carefully limited to those women in whom reliable pretherapeutic characterization of ovarian tumors can obviate the need for radical surgery.7 A woman undergoing pelvic MRI is asked about her hormonal status (day of menstrual cycle) as the ovaries and functional cysts vary in size in relation to the menstrual cycle. Information about prior gynecologic operations is also important for image interpretation. The adnexa can be imaged at intermediate field strength; however, a 1.5-T scanner is preferred if available. Bladder emptying before scanning will reduce the likelihood of motion artifact. The patient is placed in a comfortable supine position, with a foam pad to elevate the knees if desired. Unless contraindicated, an antispasmodic agent is injected intravenously (butylscopolamine or glucagon) to reduce peristalsis. A high-resolution phased-array surface coil (with at least four elements) is used for read-out.8 The coil is applied with slight pressure above the pubic symphysis to restrict abdominal wall and respiratory motion. Respiratory artifacts are further reduced by asking the patient to breathe quietly during acquisitions. The standard adnexal protocol comprises axial and coronal sequences. Axial images allow reliable evaluation of the relationship of a pelvic mass to the iliac vessels, the pelvic sidewalls, the bladder, and the rectum. Coronal images facilitate identification of the site of origin of a mass in the ovarian fossa. This standard protocol is occasionally supplemented by sagittal images to determine the relationship of a mass to the uterus, rectum, and rectouterine pouch. Both T1w and T2w sequences are needed to characterize an ovarian mass. The MRI protocol (Tables 13.1 and 13.2) should ideally begin with an axial fat-suppressed inversion recovery (IR) sequence (which requires an inversion delay of ca. 150 ms at 1.5 T). Although spatial resolution is limited, this pulse sequence will sensitively demonstrate cystic components, thereby providing important information for tissue characterization in conjunction with the nonenhanced T1w SE sequence to be performed next. Coronal T2w TSE images provide excellent resolution and high contrast for assessing the architecture of an adnexal mass. Contrast-enhanced series are acquired in two planes (axial and coronal) using a fat-suppressed T1w SE or TSE sequence. GRE sequences are inadequate for characterizing an adnexal mass due to poor contrast. A dynamic contrast-enhanced study does not add diagnostic information since most adnexal tumors are cystic or mixed cystic and solid. Single-shot TSE or HASTE sequences afford high contrast and eliminate artifacts but their spatial resolution is markedly lower than that of a T2w TSE sequence. Their use should be limited to those rare cases where T2w TSE images are suboptimal.9
M. Reuter and M. Lorenzen
Introduction
Indications
Imaging Technique
Imaging Planes
Pulse Sequences
| Sequence | Plane | Comment |
| T2w IR | Axial | Sensitive tumor detection, tissue characterization, distance from pelvic sidewall, involvement of bowel and posterior bladder wall, presence of peritoneal implants, lymph node metastasis, ascites |
| T2w TSE | Coronal | Tumor architecture, tissue characterization, delineation from uterus, vagina, vessels; presence of ascites |
| T1w SE or TSE | Axial | Tissue characterization |
| T1w SE or TSE, fat-suppressed after Gd-basedcontrast | Axial | Tumor architecture, tissue characterization |
| T1w SE or TSE, fat-suppressed after Gd-based contrast | Coronal | Tumor architecture, tissue characterization |
Contrast Media
Negative intestinal contrast medium is helpful in reliably differentiating cystic tumor components from fluid-containing bowel loops.6 To effectively suppress signal from the bowel lumen in the true pelvis, patients should start drinking the contrast solution at least 2 h before MRI is performed. An effective negative contrast solution can be prepared by dissolving one tablet of an iron gluconate formulation approved for treating iron deficiency in 1 L of water, of which the patient is asked to drink about one quarter every 30 min starting 2 h before the examination.
Features relevant for differentiating benign from malignant ovarian masses such as subtle wall irregularities, septa, and nodules often only become apparent on images acquired after administration of nonspecific Gd-based contrast medium. This is why the adnexal protocol comprises contrast-enhanced T1w SE or TSE sequences in two planes perpendicular to each other, unless the precontrast images reliably identify a hemorrhagic cyst (endometriotic or chocolate cyst) or teratoma.

MRI Appearance of Normal Anatomy
Basic Anatomy
The uterine adnexa consist of the ovaries, uterine tubes, and broad ligament. The broad ligament of the uterus is a double layer of peritoneum extending from the sides of the uterus to the lateral walls and floor of the pelvis. Its largest part forms the mesentery of the uterus (mesometrium). The superior portion of the broad ligament encasing the uterine tube on either side is the mesosalpinx. Between the layers of the broad ligament and attached to the uterus just below the uterotubal junction lie the ligament of the ovary posterosuperiorly and the round ligament of the uterus anteroinferiorly. The ovarian ligament attaches the ovary to the uterus, and the round ligament extends into the inguinal canal. The connective tissue of the broad ligament conveys the ovarian artery and vein, nerves, and lymphatic vessels.
A uterine tube (fallopian tube or salpinx) is 8–20 cm long and is supported by the mesosalpinx. It is divisible into four parts: the infundibulum—the funnel-shaped distal end that is not directly attached to the ovary but opens into the peritoneal cavity through the abdominal ostium; the ampulla—the widest and longest part; the isthmus—the thick-walled part that enters the uterine horn; and the uterine part—the short intramural segment that passes through the wall of the uterus. The abdominal ostium is surrounded by fringed processes, the fimbriae, the longest of which is attached to the ovary.
The ovaries are almond-shaped organs, each ca. 2.5–5 cm long and 0.5–1 cm thick. They have a connective tissue capsule that adheres firmly to the outer layer of modified epithelium of the peritoneal fold by which it is enveloped. The capsule encloses the ovarian cortex, the site of follicular maturation. The inner region, the ovarian medulla, has an irregular interface with the cortex and contains highly tortuous blood vessels, lymphatics, and nerves, which enter the ovary at the hilum.
During follicular maturation, primordial germ cells migrate into the ovary and develop into oogonia, which become surrounded by follicular epithelium and continue to develop into secondary follicles. Some of the secondary follicles become graafian follicles. The cells surrounding the follicle form the matrix for the follicular theca, which produces estrogen after ovulation.
In the first year of life, the ovaries and uterine tubes migrate from the thoracolumbar junction into the ovarian fossae, depressions in the pelvic sidewall bounded by the obliterated umbilical arteries anteriorly and the ureters posteriorly. The ovaries are usually lodged in the ovarian fossae in nulliparous women, but they may be displaced posteriorly into the rectouterine pouch (pouch of Douglas, cul-de-sac) or anteriorly toward the abdominal wall during pregnancy, where they may persist after delivery.
The ovaries derive their blood supply from the ovarian arteries and ovarian branches of the uterine arteries. The paired ovarian arteries arise from the abdominal aorta and pass through the retroperitoneum and the ovarian suspensory ligament to give off branches which pierce the ovaries. The uterine arteries arise from the internal iliac arteries and give off ovarian and tubal branches, which course along the attachment of the broad ligament at the lateral edge of the uterus toward the uterotubal junction and also anastomose with the ovarian arteries. Veins leaving the hilum of the ovary form a vinelike network of vessels, the pampiniform plexus. The ovarian vein formed by this plexus empties into the renal vein on the left side and directly into the inferior vena cava on the right. Blood from the uterine veins drains into the internal iliac veins.
Lymphatic drainage from the ovaries and uterine tubes is to the aortic lymph nodes in the lumbar region. Lymphatics from the region of the uterotubal junction pass through the suspensory ligament toward the superficial inguinal lymph nodes in the groin.
MRI Appearance
The normal uterine tubes are not routinely seen on MRI. In rare instances, they are depicted as horizontally oriented, tubular structures of intermediate signal intensity on T2w images.
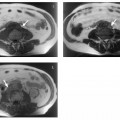
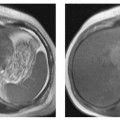
During the reproductive years, the ovaries are regularly seen on T2w images. The ovarian cortex contains the characteristic rounded, hyperintense follicles (Fig. 13.1). The size of these cystlike structures ranges from a few millimeters to 2.5 cm and varies during the menstrual cycle. The largest follicle is also known as the dominant follicle. Persistence of the dominant follicle can give rise to a follicular cyst. The latter is a functional cyst and can be differentiated from normal ovarian follicles on serial images from different phases of the menstrual cycle. Normal ovarian follicles are characterized by regular changes in size and signal intensity (Fig. 13.2). Serial examinations should be performed using TVUS. On MRI, ovarian follicles are difficult to identify on T1w images because they are isointense to muscle. Ovarian zonal anatomy, i.e., the depiction of the cortex and medulla as two distinct regions, is best appreciated on T2w images obtained before ovulation. The cortex is typically isointense to muscle, while the medulla is slightly hyperintense (intermediate in signal intensity between muscle and fat). Zonal anatomy varies during the menstrual cycle. Outwater et al. were able to identify ovarian zonal anatomy in 85 % of premenopausal women and 28% of postmenopausal women.10 Postmenopausal ovaries are less conspicuous. They have lower T2 signal intensity, which is due to atrophic fibrosis and a decrease in the number and size of follicles.

Fig. 13.1 Normal appearance of both ovaries in a 27-year-old woman. Axial T2w TSE image depicts multiple high-SI follicles.
MRI Appearance of Pathologic Entities
Pelvic Inflammatory Disease (PID)
Most cases of pelvic inflammatory disease in women result from infection with Chlamydia trachomatis or Neisseria gonorrhoeae, which ascend through the cervix and uterus, from where they can spread to the uterine tubes and ovaries.
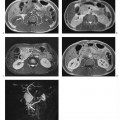
Tubal blockage secondary to salpingitis is usually not detected with routine MRI techniques but can be ascertained using MR hysterosalpingography (Fig. 13.3). With this technique, tubal patency is assessed by acquiring a 3D MR angiography sequence following instillation of diluted Gd-based contrast medium solution (for intravenous injection) into the endometrial cavity.11 Early salpingitis is suggested by fluid retention in the uterine tubes, which would not be identified otherwise. Coronal T2w images are well suited for this purpose because they depict the tubes as high-signal-intensity structures extending nearly horizontally from the uterus to the ovaries. The MR morphology of pyosalpinx is indistinct from that of uncomplicated tubal dilatation. Tubo-ovarian abscess is a complication of inflammation of the serosal coat of the ovaries secondary to spread of salpingitis into the rectouterine pouch. An abscess is depicted by MRI as edema around the ovary, which is usually not enlarged.
Ectopic Pregnancy
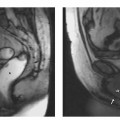
Ectopic pregnancy is diagnosed using TVUS, which can detect 62 % of all ectopic pregnancies as early as 7 weeks of gestation. MRI is rarely indicated in early pregnancy, which is why ectopic pregnancy is usually an incidental finding at MRI. The MR appearance is that of a cystlike mass of predominantly high signal intensity. Solid or necrotic areas of intermediate signal intensity may be present, depending on whether or not the product of conception is intact. If an ectopic pregnancy is suspected at MRI, a sagittal sequence should be acquired for better delineation of the chorionic cavity. Confident differentiation from cystic adnexal tumors is not always possible.
Ovarian Torsion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree