Chapter 18 The Pleura
PLEURAL EFFUSIONS
General Considerations and Clinical Features
Pleural effusions occur when the rates of entry and exit for pleural liquid and protein are no longer in equilibrium. Increased pleural fluid may result from one of six mechanisms (Box 18-1):
Pleural effusions may be transudates or exudates (Box 18-2). Transudates are usually caused by increased capillary hydrostatic pressure or decreased osmotic pressure, as in CHF, hypoalbuminemia, hepatic cirrhosis, and nephrotic syndrome. Management of transudates usually consists of treatment of the underlying cause, such as CHF. Exudates result from inflammatory and neoplastic processes involving the pleura. Other examples include pulmonary infarction and collagen vascular diseases.
Radiologic Features
Standard Radiography
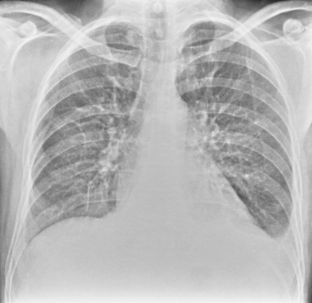
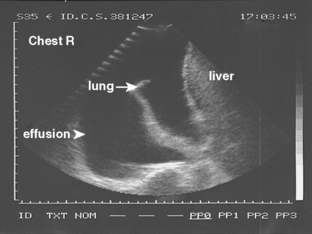
On an upright chest radiograph, a free pleural effusion demonstrates a meniscus sign, which is a concave, upward-sloping interface with the lung that causes sharp or indistinct blunting of the costophrenic angle (Figs. 18-1 and 18-2). Approximately 200 mL of fluid usually is necessary to blunt the lateral costophrenic angle, although smaller amounts (>75 mL) can produce a meniscus that blunts the posterior costophrenic angle on the lateral view (Fig. 18-3). A lateral decubitus view of the chest is much more sensitive than the upright view in the detection of pleural effusion, and as little as 5 mL of fluid can be demonstrated on decubitus views (Fig. 18-4).
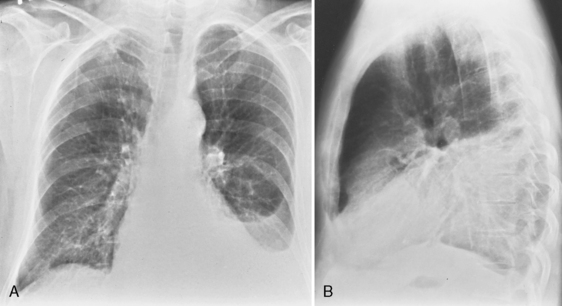
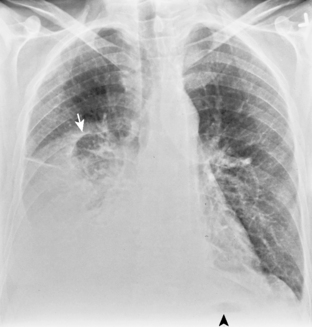
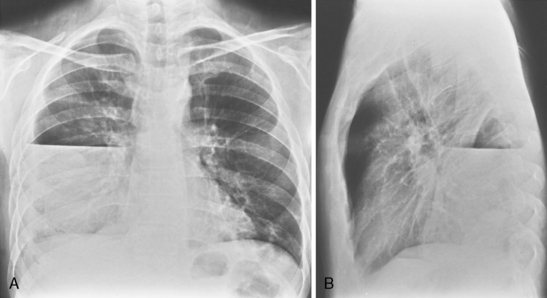
In the upright position in the normal individual, a small amount of pleural fluid accumulates in a subpulmonic position. In certain individuals, a large amount of free-flowing pleural fluid may accumulate in this position before spilling into the costophrenic angles. On the frontal view, this produces a characteristic appearance with elevation of the apparent ipsilateral hemidiaphragm, flattening of the medial aspect, and displacement of the peak of the apparent diaphragm laterally. On the left side, this is easy to recognize because of separation of the stomach bubble from the apparent left hemidiaphragm (Fig. 18-5). A massive effusion produces a complete or nearly complete opacification of a hemithorax, with displacement of the mediastinum to the opposite side (Fig. 18-6). This appearance contrasts with complete atelectasis of the lung, in which the shift of the mediastinum is toward the side of the opaque hemithorax. Moderate to large amounts of pleural effusion may be missed on supine radiographs. These effusions layer posteriorly and produce a generalized increase in opacity of the hemithorax, through which the pulmonary vessels can be visualized (Fig. 18-7). There may be blunting of the costophrenic angle, and the fluid occasionally tracks over the apex of the lung, producing an apical cap.
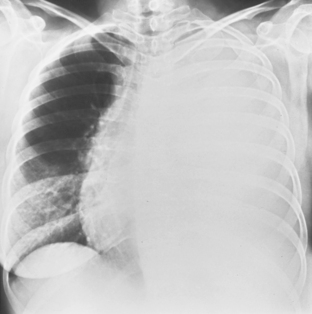
Figure 18-6 Massive effusion. There is a mediastinal shift to the opposite side and a completely opaque left hemithorax.
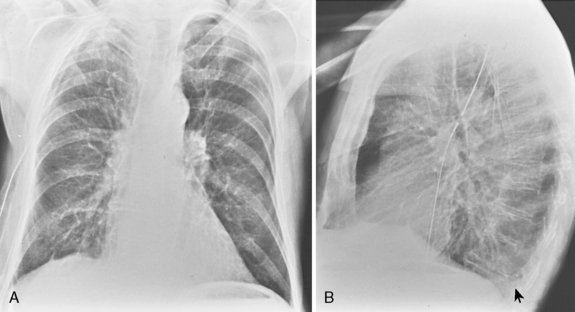
Fluid may occasionally accumulate within fissures, and these accumulations may produce the appearance of a mass or pseudotumor (Fig. 18-8). Differentiation from a mass can be easily made because the fluid is free and shifts on decubitus views.
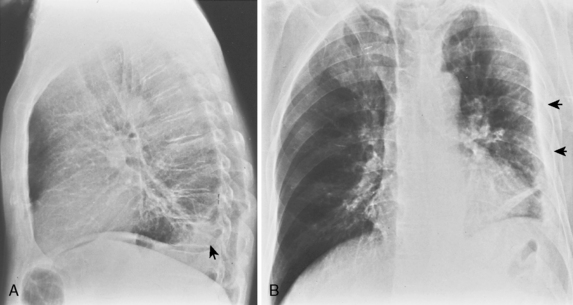
Pleural effusions frequently loculate (Fig. 18-9); i.e., they do not shift freely in the pleural space because of adhesions between the visceral and parietal pleura. Loculation of fluid occurs in exudative effusions, particularly in patients with empyema or hemothorax.
Ultrasound
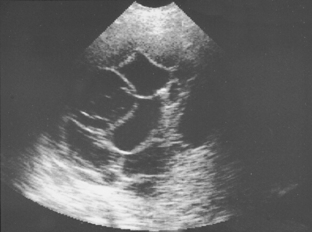
Pleural fluid collections may be anechoic or echoic, and they may change shape during respiration. Most collections are anechoic (Fig. 18-10) and are delineated by an echogenic line of visceral pleura and lung. Anechoic effusions are usually transudates, whereas effusions that contain septations represent exudates in approximately 80% of cases (Fig. 18-11).
Computed Tomography
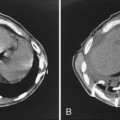
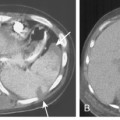
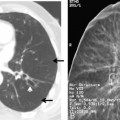
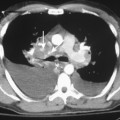
Free-flowing pleural fluid produces a sickle-shaped opacity in the most dependent part of the thorax posteriorly on CT scanning (Fig. 18-12). CT allows very small amounts of pleural fluid to be detected. Loculated fluid collections are seen as lenticular opacities in fixed positions (see Fig. 18-9). CT is of limited value in differentiating transudates from exudates or in the diagnosis of chylous pleural effusions. Although most chylous effusions are indistinguishable from other causes of pleural effusion on CT, low attenuation consistent with fat or a fat-fluid level in the pleural collection is rarely seen. Acute pleural hemorrhage, however, can be identified by the presence of a fluid-fluid level or by increased attenuation of the pleural fluid collection (Fig. 18-13).
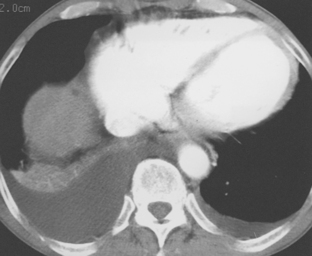
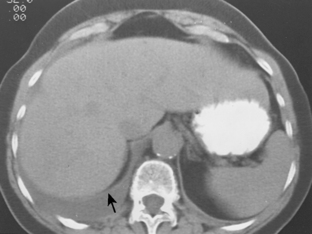
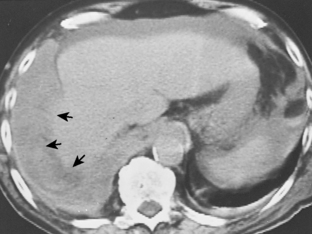
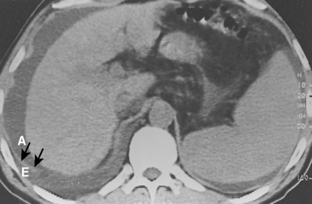
Pleural fluid can be distinguished from ascites by several CT features (Box 18-3), including the displaced crus sign, the interface sign, the diaphragm sign, and the bare area sign. If the diaphragmatic crus is displaced away from the spine by an abnormal fluid collection, the fluid is located in the pleural space (Fig. 18-14). The location of ascites is lateral and anterior to the crus. The interface sign describes a sharp interface that can be identified between fluid and the liver or spleen when ascites is present. In cases of ascites, the interface is sharp, whereas in cases of pleural effusion, the interface is ill defined (Fig. 18-15). If the diaphragm is identifiable adjacent to an abnormal fluid collection in the right upper quadrant, the diaphragm sign is probably the most reliable means of differentiating fluid from ascites (Fig. 18-16). The location of the diaphragm is readily visible in patients with ascites but may not be identified in patients with pleural effusions. Pleural effusion is visualized outside the hemidiaphragm, whereas ascites is seen within the hemidiaphragmatic contour. The bare area is the portion of the right lobe of the liver that lacks peritoneal covering. Restriction of peritoneal fluid by the coronary ligaments from that area is another useful distinguishing sign. To differentiate pleural effusions from intra-abdominal fluid collections, all four signs should be assessed in each case. The attempt to differentiate ascites from pleural effusion may have pitfalls. A large pleural effusion, particularly on the left side, may cause inversion of the diaphragm, resulting in the pleural fluid being located centrally rather than peripherally.
Box 18-3 Computed Tomography: Pleural Fluid versus Ascites
| Pleural Fluid | Ascites |
|---|---|
| Displaced crus | |
| Ill-defined interface with liver and spleen | Sharp interface with liver or spleen |
| Fluid outside diaphragm contour | Fluid inside diaphragm contour |
| Fluid excluded from bare area of liver |
CT is helpful in the assessment and management of loculated pleural effusions (see Fig. 18-9). Accurate localization of loculated collections is useful before drainage. Loculated effusions have a lenticular configuration with smooth margins, and they displace the adjacent parenchyma. This typical appearance for any pleural process is a distinguishing feature that can help differentiate a pleural from parenchymal process.
Magnetic Resonance Imaging
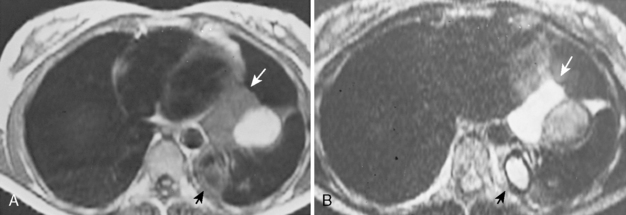
The role of MRI in the evaluation of the pleura is somewhat limited. MRI does provide certain advantages because of its ability to image the thorax directly in the axial, sagittal, and coronal planes. MRI may be slightly superior to CT in the characterization of pleural fluid (Fig. 18-17). Typically, fluid collections in the pleural cavity have a low signal intensity on T1-weighted images and relatively high signal intensity on T2-weighted images because of the water content. It may be possible to differentiate transudates, simple exudates, and exudates with the use of a triple-echo pulse sequence. Complex exudates have greater signal intensity than simple exudates, which are brighter than transudates. Preliminary results also suggest chylothorax may have distinctive findings on MRI, with signal intensity characteristics similar to those of subcutaneous fat. Subacute or chronic hematomas demonstrate typical signal intensity on MRI with a concentric ring sign, consisting of an outer dark rim composed of hemosiderin and bright signal intensity in the center because of the T1 shortening effects of methemoglobin.
EMPYEMA
Clinical Features
An empyema is an exudative effusion with pus in the pleural cavity. Several criteria are used for the diagnosis of empyema (Box 18-4): grossly purulent fluid; organisms identified on the basis of Gram stain or culture; a white blood cell count in the pleural fluid greater than 5 × 109 cells/L, and a pH below 7 or glucose level less than 40 mg/mL. The natural progression of an empyema consists of several phases: from an exudative to a fibropurulent phase to an organizing phase that results in the development of a thickened pleura, or fibrin peel. Early diagnosis and treatment is therefore imperative.
Box 18-4 Fluid Characteristics of Empyema
Organisms identified by stain or culture
White blood cell count > 5 × 109 cells/L
Radiologic Features
The radiologic features of empyema are described in Box 18-5.
Box 18-5 Radiologic Features of Empyema
STANDARD RADIOGRAPHY
Air-fluid level (bronchopleural fistula) varies in length on posteroanterior and lateral radiographs
Standard Radiography
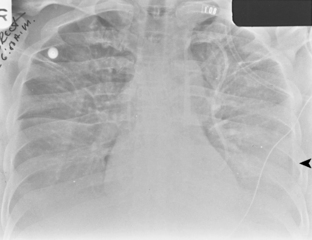
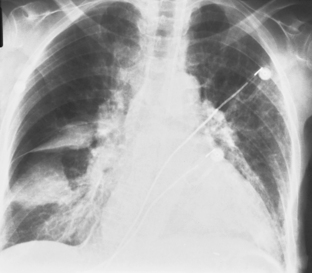
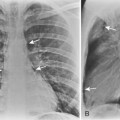
Most empyemas manifest as a classic pleural effusion. However, they tend to loculate early and, as a result, may not change with the patient’s position, or they may not have a classic meniscus sign. Loculated collections have a lenticular shape that forms obtuse angles with the chest wall. If a bronchopleural fistula is present, an air-fluid level can be identified in the empyema space before thoracentesis (Fig. 18-18). On standard radiographs, the length of the air-fluid level varies on radiographs taken at 90 degrees to each other; there may be a short air-fluid level on the frontal radiograph and a long air-fluid level on the lateral radiograph. This finding contrasts with lung abscesses, in which the length of the air-fluid level is usually equal on both views.
Computed Tomography
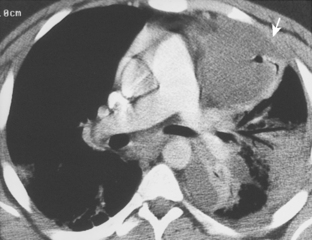
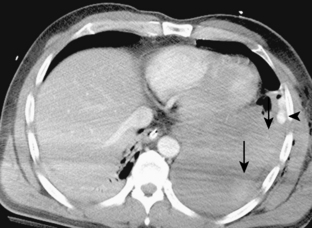
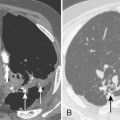
CT is particularly helpful in establishing the diagnosis of empyema and in differentiating empyemas from lung abscesses. The most reliable sign is the split pleura sign, which is usually identified during the organizing phase of an empyema (Fig. 18-19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree