12 The Uterus and Vagina As the imaging modality with the highest sensitivity and specificity for many benign and malignant diseases of the female pelvis, MRI has an increasingly important role in women’s imaging. Evaluation of the uterus benefits from the excellent multiplanar capability of MRI (e. g., in the sagittal plane), while the high intrinsic soft-tissue contrast is unsurpassed in depicting uterine zonal anatomy. New coils and fast pulse sequences ensure high image quality and have further contributed to the evolution of MRI into a powerful diagnostic tool for imaging the female pelvis. Although ultrasound secondary to a pelvic examination continues to be the most widely used imaging tool, it has several well-known limitations such as being examiner dependent and providing poor tissue characterization. The most notable drawback in the setting of female pelvic malignancy is the fact that it does not allow adequate tumor staging. CT offers inadequate soft-tissue contrast for reliable local tumor staging and should only be used in women with advanced pelvic cancer. MRI, on the other hand, visualizes the female pelvis with exquisite morphologic detail and is the most accurate imaging modality for tumor staging. Recent studies have also shown MRI to be a cost-effective test for the pretherapeutic staging of cervical and endometrial cancer. Finally, MRI is increasingly being used for diagnostic work-up and therapeutic decision-making in women with benign uterine disease. MRI is the diagnostic test of choice for evaluating malignant tumors of the uterus (cervical and endometrial cancer) and carcinoma of the vagina. It is increasingly being used for planning radiotherapy and monitoring the response to treatment. As an adjunct modality, MRI can help resolve inconclusive ultrasound findings, determine the site of origin of a pelvic mass, and provide useful information for lesion characterization. A woman undergoing a pelvic MRI examination should be asked not only the standard questions regarding known contraindications to MRI (pacemaker, etc.) but also a set of questions pertaining to her gynecologic history and hormonal status. This information is important for tailoring the pelvic MRI study to the individual situation and will help the radiologist to adequately interpret the results since the appearance of the pelvic organs varies through the menstrual cycle and with the patient’s hormonal status. Also important is information on the presence of foreign bodies, such as an intrauterine device (IUD), and previous surgery and radiotherapy. We use a special form for the patient’s gynecologic data, so the information is available at a glance when the radiologist interprets the MR images (Table 12.1). Earlier fears that IUDs might lead to overheating or become displaced have proved unfounded. An IUD is no longer considered a contraindication to MRI, which will depict the device as a low-signal-intensity structure in the uterine cavity. It is unnecessary for patients to fast before undergoing pelvic MRI. The bladder should be empty or only moderately distended at the beginning of the examination to minimize motion artifacts and avoid the need to interrupt the examination if the patient has an urge to void. Placement of a tampon for improved delineation of the vaginal wall is not necessary and may even cause susceptibility artifacts on GRE sequences. Pelvic MRI can be performed with the patient in the prone position (which will reduce respiratory artifacts) or in the preferred and more comfortable supine position. Various measures are available to reduce ghosting and blurring resulting from respiratory motion, intestinal peristalsis, and vessel pulsation. Respiratory artifacts from the abdominal wall can be minimized by instructing the patient to breathe quietly during data acquisition. If MRI is performed with the patient supine, it is helpful to place a presaturation band over the anterior abdominal wall to reduce the signal from abdominal fat (Figs. 12.1 and 12.2). Unless contraindicated, intestinal peristalsis can be effectively suppressed by intravenous split-dose administration of a spasmolytic agent (40 mg of butylscopolamine or 2 mg of glucagon). The venous line can also be used for later injection of contrast medium. Presaturation pulses applied superior and inferior to the field of view (FOV) reduce arterial and venous inflow artifacts (Fig. 12.1). An anteroposterior phase-encoding direction should be chosen for acquisition of axial (and sagittal) images to move vascular pulsation artifacts away from the target anatomy in the center of the pelvis.
C. Kluener and B. Hamm
Introduction
Indications
Imaging Technique
| 1. What is the patient’s menstrual status? | ||
| Premenopausal | Postmenopausal | |
| Date of last period: Is pregnancy possible? | ||
| No | Yes | |
| 2. Does the patient take hormones? | ||
| Estrogens: | No | Yes |
| Oral contraceptives: | No | Yes |
| 3. Parity? | ||
| Nullipara | Multipara | Cesarean |
| 4. Presence of foreign bodies (e.g., tampon, IUD, cervical pessary)? | ||
| No | Yes | |
| If yes, specify: | ||
| 5. Did the patient have prior gynecologic surgery (including D&C) or radiotherapy? | ||
| Surgery: | No | Yes |
| If yes, type and date of operation: | ||
| Radiotherapy: | No | Yes |
| If yes, date of completion: | ||
| 6. Does the patient have contraindications to | ||
| Contrast media? | No | Yes |
| Butylscopolamine? | No | Yes |
| Glucagon? | No | Yes |

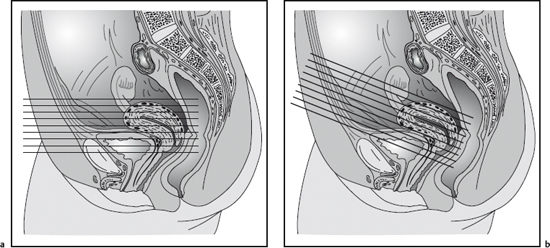
Fig. 12.1 Sagittal T1w GRE image showing placement of presaturation bands on the high-SI abdominal wall for reduction of motion artifacts and of presaturation bands superior and inferior to the imaging volume for elimination of pulsation artifacts.
Although pelvic MRI can be performed with the inbuilt body coil, use of a body or torso phased-array coil is now preferred because it markedly improves signal-to-noise ratio (SNR) and spatial resolution (Fig 12.3). Paired coils in Helmholtz arrangement and endoluminal coils are no longer used for pelvic imaging. An endorectal coil images nearby structures with high resolution (e. g., cervical cancer) but does not improve overall staging accuracy and is not well accepted by patients.
Imaging Planes
Proper selection of imaging planes is critical in planning a pelvic MR examination. Axial images enable adequate evaluation of the uterus and cervix, uterosacral ligaments, and presacral space and are optimal for parametrial and lymph node assessment. Sagittal images cover the entire length of the uterus, which is an advantage over axial images, and they are more suitable for evaluating the vesicouterine ligament and the anatomic relationship of the uterus and vagina to the urinary bladder and rectum. Sequence planes angled to the uterus or cervix to obtain true long-axis and short-axis views of these organs are helpful in assessing uterine anomalies (long-axis view) and depth of myometrial invasion in endometrial cancer (short-axis view of the uterine corpus). Otherwise, additional coronal images are rarely acquired in uterine and vaginal MRI.
Pulse Sequences
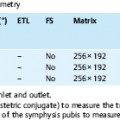
The protocol for MRI of the uterus and vagina comprises T1w and T2w sequences. T2w images best depict the zonal anatomy of the uterus and are most suitable to evaluate the integrity of muscular structures (e. g., bladder and rectal wall, pelvic sidewall). T1w sequences provide optimal contrast between pelvic organs and surrounding fat, making them well suited for lymph node assessment. Imaging parameters for conventional T1w and T2w SE sequences as well as their fast counterparts (FSE or TSE) are summarized in Table 12.2. Part of the gain in imaging speed resulting from the use of TSE or FSE sequences should be used to increase the imaging matrix and number of signal averages. Combining an FSE or TSE sequence with the use of a phased-array body coil yields images with an excellent spatial resolution while also shortening acquisition time. Fat-suppressed SE sequences will help differentiate between hemorrhagic and fatty lesions (e. g., endometriosis cyst vs dermoid) and improve detection of small, hyperintense endometrial implants, which may escape detection on conventional images when surrounded by hyperintense fat. A fat suppression technique also improves the differentiation of fat from enhancing tissue on postcontrast images.

Fig. 12.2a, b Improved image quality resulting from use of presaturation pulses. T1w SE images obtained without and with abdominal presaturation. a Without presaturation, respiratory artifacts from the high-SI abdominal wall degrade the image through the true pelvis. b Presaturation of the abdominal wall eliminates respiratory motion artifacts. Normal appearance of the ovaries in the ovarian fossae. Sharp visualization of the intestine due to administration of spasmolytic agent.

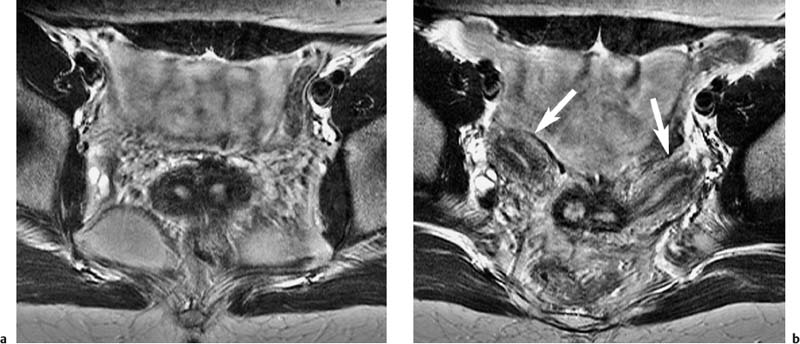
Fig. 12.3a, b Improved SNR resulting from use of a phased-array body coil. T2w TSE images obtained with identical sequence parameters. Images through the endometrial cavity showing intramural fibroids. a Body coil. b Phased-array body coil.
Because of their poorer spatial resolution, gradient echo (GRE) sequences have not established themselves as an alternative MR technique for imaging of the female pelvis. They are occasionally used for dynamic contrastenhanced MR studies.
Contrast Media
After intravenous injection of a nonspecific, Gd-based contrast medium (e. g., Magnevist, Dotarem), the uterine zonal anatomy is also seen on T1w images (see Figs. 12.13 and 12.19), but less clearly so than on T2w images. A contrast-enhanced study is generally performed using a high-resolution T1w SE sequence, but is only required for specific indications (e. g., endometrial cancer, differentiation of viable tumor and necrosis).
Routine administration of an oral contrast agent is not necessary for a dedicated MRI examination of the uterus or vagina.
General Imaging Strategy
The basic MRI protocol for the uterus comprises a sagittal T2w sequence and axial T2w and T1w sequences. This is the standard combination of pulse sequences recommended for evaluating uterine anomalies as well as benign and malignant tumors (e.g., fibroids, endometrial and cervical cancer). Sagittal images are acquired to detect endometrial cancer and define its longitudinal extent as well as to identify possible invasion of the urinary bladder and rectum. Axial T2w images are most useful to evaluate for invasion of the parametrium, sacrouterine ligaments, and pelvic sidewall. Axial images also provide supplementary information for assessment of the urinary bladder and rectal wall. As a rule, T1w images (from the aortic bifurcation to the pelvic floor) are only acquired in the axial plane; they are most useful for assessing the lymph node status. When evaluating women for uterine anomalies, the standard orthogonal planes should be replaced with oblique images oriented parallel to the endometrial cavity to obtain true long-axis views of the uterus (Fig. 12.4). In patients with endometrial cancer, additional contrast-enhanced T1w images in sagittal and oblique axial planes (short-axis view of uterus) will increase diagnostic confidence.
An axial T2w sequence is the basic sequence for MRI of the vagina, with axial T1w images providing useful additional information (see Fig. 12.21). After contrast administration, zonal anatomy is also seen on T1w images (see Fig. 12.21c). An additional sagittal T2w sequence is useful to determine the longitudinal extent of a vaginal tumor and demonstrate invasion of the urinary bladder and rectal wall. A coronal sequence (T2w) is indicated if invasion of the levator ani muscle is suspected.
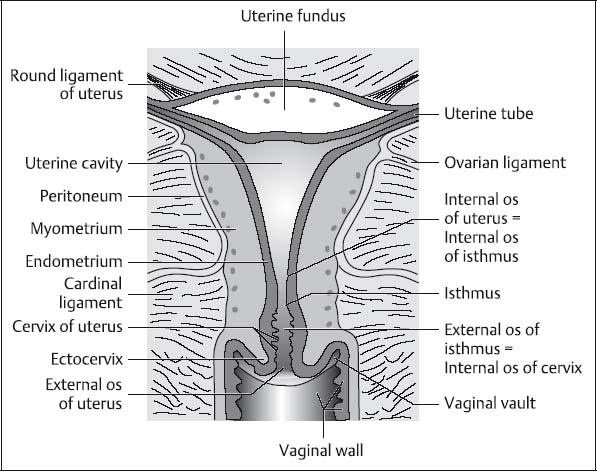
Fig. 12.5 Diagram of the uterus showing the corpus, isthmus, and cervix (according to Martius).
MRI Appearance of Normal Anatomy
The normal MRI appearance of the uterus and vagina varies with the patient’s age and hormonal status.1–6
The uterus can be divided into three distinct segments—the corpus, the isthmus (or lower uterine segment), and the cervix (Fig. 12.5).
The wall of the uterine corpus (unlike that of the cervix) is predominantly composed of smooth muscle. The slitlike uterine cavity is lined by endometrium. In the nonpregnant uterus, the isthmus, together with the internal os, constitutes the junction of the uterine corpus and the cervix. The isthmus is only ca. 0.5 cm long but is quite distinct from the cervix in that it enlarges considerably during pregnancy to become the lower part of the uterine cavity. The uterine cervix consists of supravaginal and vaginal parts. The rounded vaginal part (ectocervix or portio vaginalis cervix) projects into the vagina. The wall of the cervix is mostly composed of firm connective tissue and ca. 10 % smooth muscle fibers arranged in a ringlike fashion. The cervical canal is lined by columnar epithelium and the ectocervix by stratified squamous epithelium. The transition between these two types of epithelium is at the level of the external os.

Fig. 12.6 Sagittal T2w TSE image of the uterus showing the parts of the uterus covered by peritoneum (white line). The peritoneal cavity in the true pelvis appears black.
The uterus is covered by peritoneum (Fig. 12.6). Anteriorly, the peritoneum is reflected from the uterus onto the superior surface of the urinary bladder, creating the vesicouterine pouch. Posteriorly, the peritoneum extends down to the level of the posterior vaginal fornix, from where it is reflected onto the anterior rectal wall, forming the rectouterine pouch (pouch of Douglas, cul-de-sac). The uterine vessels pass through the parametrium and reach the uterus at the level of the uterine isthmus (i. e., at the internal os of the cervical canal). The ureters course through the parametrial tissue on either side ca. 2 cm lateral to the cervix. The parametrium is mainly composed of connective tissue with a larger proportion of fatty tissue toward the pelvic sidewalls. Below, the parametrium is separated from the paravaginal connective tissue (the paracolpium) by the cardinal ligaments (Mackenrodt ligaments), which extend from the cervix to the pelvic sidewalls. The uterus is supported and suspended by a total of eight ligaments. The uterovesical and uterosacral ligaments are important landmarks, for example, when evaluating the extent of cervical carcinoma (Fig. 12.7). The uterovesical ligament extends from the cervix to the base and posterior surface of the urinary bladder, while the uterosacral ligament extends from the cervix to the sacrum, surrounding the rectum.
In women of reproductive age, the entire uterus is 6–9 cm long. It has uniform low signal intensity on T1w images (Fig. 12.8), whereas three distinct zones—the endometrium, the junctional zone, and the myometrium—can be recognized on T2w images (Fig. 12.9).

Fig. 12.7 Ligaments of the internal female genital organs (according to Martius).
The endometrium is of high T2 signal intensity. Its thickness varies during the menstrual cycle from 1–3 mm at the beginning of the proliferative phase (Fig. 12.10) to 5–7 mm in the mid-secretory phase (Figs. 12.9 and 12.11). A blood clot may occasionally be present in the endometrial cavity at the time of menstruation and must not be mistaken for a foreign body or pathology (Fig. 12.12).
The outer myometrium is of intermediate signal intensity on T2w images and increases in signal intensity during the secretory phase due to a higher water content (compare Figs. 12.9 and 12.11 with Fig. 12.10). Myometrial vessels are also prominent during this menstrual phase. The junctional zone represents the innermost myometrium and is of low signal intensity because it has a lower water content and higher nucleus-to-cytoplasm ratio than the outer myometrium.7,8 A thickness of the junctional zone of up to 5 mm is normal, while a thickness > 12 mm is considered to be diagnostic of adenomyosis.
Following intravenous injection of a paramagnetic, Gd-based contrast medium, uterine zonal anatomy is also visualized on T1w images (Fig. 12.13). There is marked enhancement of the endometrium and myometrium, whereas the junctional zone remains low in signal intensity, which has been attributed to its more compact structure and smaller extracellular space for distribution of the contrast agent.8,9

Fig. 12.9 Normal zonal anatomy of the uterus in mid-cycle in a 30-year-old woman. T2w SE image shows high-SI endometrium surrounded by low-SI junctional zone. The outer myometrium is of moderately high SI.

Fig. 12.10 Uterus in the first half of the menstrual cycle (proliferative phase) in a 32-year-old woman. T2w SE image shows thin endometrial layer and rather low SI of myometrium.
On MRI of the neonatal uterus, the myometrium and endometrium can be differentiated because of the prenatal influence of maternal hormones. Before menarche, the endometrium is indistinct or appears as a thin stripe of high signal intensity, while the junctional zone cannot be distinguished from the low-signal-intensity myometrium. At this age, the uterine corpus is small and shorter than the cervix (Fig. 12.14).

Fig. 12.12 Small blood clot (arrow) in the endometrial cavity on T2w TSE image.

Fig. 12.13 Normal appearance of the uterus after IV administration of Gd-based contrast medium. Same patient as Fig. 12.12. T1w SE image shows prominent enhancement of the endometrium and marked enhancement of the vaginal mucosa. Low SI of the vaginal muscle.

Fig. 12.14 Normal uterus in a sexually mature 14-year-old girl on T2w TSE image. Note the length of the uterine cervix relative to the length of the corpus.
In postmenopausal women, T2w images will demonstrate a thin stripe of high-signal-intensity endometrium, while the junctional zone and myometrium are indistinct because both are of low signal intensity (Fig. 12.15). The endometrial thickness is ca. 3–5 mm. In women on hormone replacement therapy, the endometrium may be up to 10 mm thick and the premenopausal signal characteristics and zonal anatomy are preserved. Ultrasonography also distinguishes three uterine layers, but these do not exactly match the zones seen on MRI, which explains the divergent thickness data.10
Hormone treatment also affects the MRI appearance of the uterus and vagina. The myometrium has higher signal intensity in women who take oral contraceptives (Fig. 12.16).
T2w images of the adult cervix show an innermost area of high signal intensity surrounded by a rather thick layer of low signal intensity and an outer layer of intermediate signal intensity (Figs. 12.11 and 12.17). The high-signal-intensity area corresponds to intracervical mucus and the endocervical glands. High-resolution MRI will occasionally depict an additional thin stripe of moderate hyperintensity (Fig. 12.18), which most likely represents the cervical mucosa. Also seen on high-resolution images are the palmate folds. The low-signal-intensity layer represents the cervical stroma, which is rich in connective tissue. In contrast, the outer layer, which is similar in signal intensity to the uterine myometrium, has a less compact structure.11
Nabothian cysts are common benign cervical lesions that develop when mucous glands become enlarged or obstructed. They are depicted on T2w images as well-defined, round or oval lesions of high signal intensity (Figs. 12.6 and 12.42).12 After intravenous contrast administration, the cervical zonal anatomy is also seen on T1w images with the cervical mucosa, the ectocervix, and the outer cervical stroma showing stronger enhancement (Fig. 12.19). The MRI appearance of the cervical zonal anatomy does not change much after menopause, nor is it much different in women taking exogenous hormones or oral contraceptives.
The parametrium has low signal intensity on T1w images, due to its large connective tissue component and the presence of blood and lymphatic vessels, and cannot be reliably differentiated from the uterine cervix (Fig. 12.20a). On T2w images, the higher signal intensity of the parametrial tissue allows good differentiation from the inner layer of low-signal-intensity cervical stroma (Fig. 12.20b) but may be indistinct from the outer cervical stroma, which is of similar T2 signal intensity. In advanced endometrial cancer with parametrial invasion, tumor in the fatty tissue near the pelvic sidewalls is best appreciated on T1w images.
The vagina is of intermediate signal intensity on T1w images, similar to that of the urethra and rectum (Fig. 12.21a). Differentiation of these structures is much better on T2w and contrast-enhanced T1w images (Fig. 12.21b and c). Insertion of a tampon into the vagina is not necessary and may even distort anatomy. Sagittal images are most suitable to differentiate the posterior vaginal fornix from the cervix and anterior rectal wall (Figs. 12.18 and 12.19), whereas the smaller anterior fornix is less well delineated from the cervix. The paravaginal tissues (the paracolpium) contain many veins and therefore have high T2 signal intensity (Figs. 12.12 and 12.21b).
The MRI appearance of the vagina, like that of the uterus, responds to hormones. In the early proliferative phase, the vagina is of low signal intensity on T2w images with a central hyperintense stripe corresponding to mucus and epithelium. During the secretory phase, the central stripe is somewhat thicker, and, in ca. 70% of women, the vaginal wall increases in signal intensity. Thus, the contrast between the wall and mucosa/mucus is reduced compared with the proliferative phase.13 In postmenopausal women not taking exogenous hormones, T2w images show a low-signal-intensity vagina with a very thin central stripe of high signal intensity. In postmenopausal women on hormone replacement therapy, the vagina has the same MR appearance as during the proliferative phase in premenopausal women.

Fig. 12.15 Postmenopausal uterus on T2w SE image.

Fig. 12.16 Normal uterus in a woman taking oral contraceptives. T2w SE image shows thin endometrium and increased myometrial SI.

Fig. 12.18 Normal appearance of the uterine cervix on sagittal T2w TSE image. Four zones can be distinguished: the innermost high-SI mucus, the palmate folds, the low-SI inner cervical stroma, and the intermediate-SI outer stroma. The ectocervix protrudes into the vagina. Good delineation of the posterior vaginal fornix (arrow) from the high-SI mucus.

Fig. 12.19 Normal appearance of the uterine cervix on sagittal T1w SE image after IV contrast administration. There is enhancement of the cervical endometrium and mucosa and of the vagina (moderate enhancement of the outer myometrium), but no enhancement of the mucus in the cervical canal and vagina. Good delineation of the posterior and anterior vaginal fornices. Very high SI of the urine in the bladder following IV contrast injection.

Fig. 12.21a–c Normal axial view of the vagina. a Poor delineation of the vagina from the paracolpium, urethra, and rectum on T1w SE image. b Good differentiation of the vagina from the high-SI paravaginal tissues, urethra, rectum, and levator ani muscle on T2w image. c Axial T1w image of the vagina after IV contrast administration.
MRI Appearance of Pathologic Entities
Congenital Uterine Anomalies
The lower part of the vagina develops from the urogenital sinus, while the uterine tubes, uterus, cervix, and upper part of the vagina derive from the paired Müllerian ducts of the embryo. Müllerian duct anomalies result from agenesis, absent or incomplete fusion of the two ducts, or failure of septal resorption (Fig. 12.22). Such anomalies are present in 2–3 % of all women14,15 and are diagnosed in 9–25 % of women presenting with infertility or a history of miscarriage.16,17 Clinical signs and symptoms of uterine anomalies comprise pain due to primary amenorrhea, recurrent miscarriage, complications during delivery, and symptoms of endometriosis. It is important to note that 20–40 % of women have concomitant renal anomalies (e. g., agenesis, ectopia, malrotation).
Besides diagnostic laparoscopy, there are several noninvasive tests such as hysterosalpingography, endovaginal ultrasound, and MRI for diagnosing uterine anomalies. MRI allows comprehensive evaluation of pelvic anatomy and also permits concomitant assessment of the entire urinary tract18,19 and is therefore considered the modality of choice, at least in women who are candidates for surgery.20–22
MRI should be performed with the highest possible spatial resolution. The use of an FSE or TSE sequence with multiple signal averages23–25 and a high matrix in conjunction with a phased-array body coil is recommended. There are no special requirements regarding patient preparation and positioning. No intravenous contrast medium is needed. The pulse sequences to be acquired (Table 12.3) are coronal T1w images with a large FOV for evaluation of the kidneys and urinary tract, followed by T2w sequences of the true pelvis in sagittal and axial planes with a slice thickness of 4–5 mm. These are the most important pulse sequences for evaluation of the uterine contour and zonal anatomy as well as of the ovaries. The axial images can be used to prescribe an oblique sequence angled to the long axis of the uterus (Fig. 12.4), which is important for differentiating a septate from a bicornuate uterus. The shape of the endometrial cavity is best appreciated when the endometrium is thick during the secretory phase. Axial T1w images additionally allow characterization of ovarian cysts, fibromas, and hemorrhage. If endometriosis is suspected, a fat-saturated T2w sequence should be obtained to identify small endometriotic deposits.
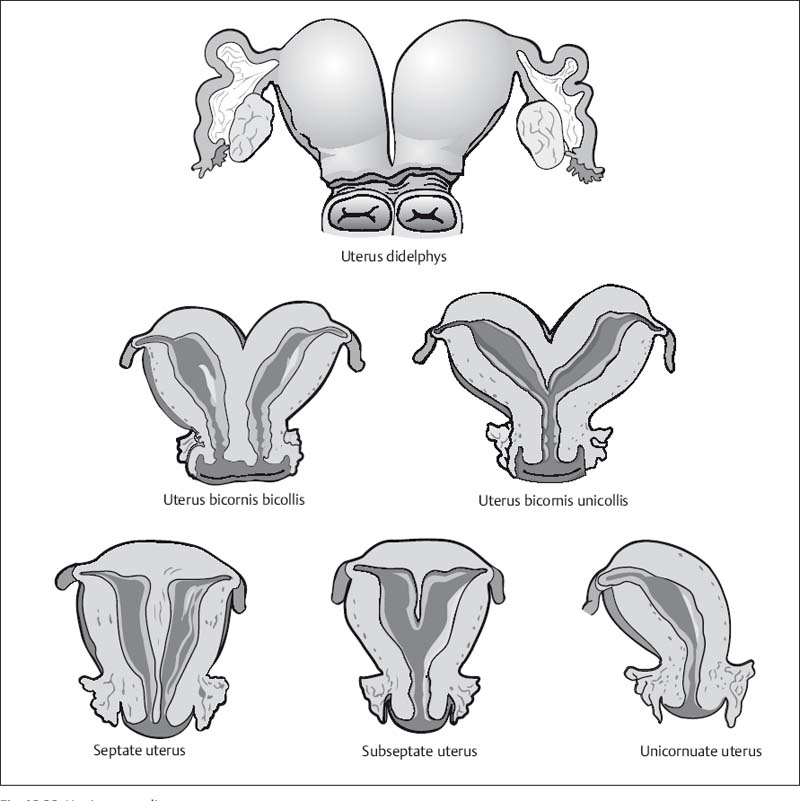
Fig. 12.22 Uterine anomalies.
MRI allows good evaluation for agenesis of the different parts of the urogenital tract, except for the ovarian tubes (Fig. 12.23). The most common form of urogenital agenesis is Mayer–Rokitansky–Küster–Hauser syndrome, which is characterized by the absence of the uterus, cervix, and upper part of the vagina, while the tubes and ovaries are normal in most cases. It is the second most common cause of primary amenorrhea after gonadal dysgenesis.26 Isolated agenesis or hypoplasia of the uterus is very rare.
Absence of the uterus is best seen on sagittal T2w images, while absence of the cervix and proximal vagina is easier to identify on axial images. In Mayer–Rokitansky–Küster–Hauser syndrome, there is prominent absence of the uterus and cervix with a blind-ending lower vagina. A hypoplastic uterus with absence of the upper vagina is rare.27
Uterus Didelphys (Double Uterus)
A uterus didelphys results from complete failure of müllerian duct fusion and is characterized by the presence of two fully developed uterine bodies and cervices. This anomaly may be associated with a longitudinal or transverse septum dividing the upper vagina. Obstruction of one of the uterine cavities by the septum results in hematometrocolpos with ipsilateral hematosalpinx.25
Although the two uterine bodies are quite far apart, the cervical canals lie side by side but are completely separate (Fig. 12.24). Whenever two cervical canals are demonstrated by imaging, special attention should be paid to the presence of a vaginal septum or a double vagina (Fig. 12.24). Since the vaginal septum is not always seen on MRI, the clinical findings must be taken into account to differentiate a uterus didelphys from a uterus bicornis bicollis.25,26
| Sequence | Scan area/FOV (plane) | Comment |
| T1w SE | Entire urinary tract (coronal) | Renal and urinary tract anomalies |
| T2w TSE | Uterus and vagina including ovaries (axial and sagittal) | Uterine anomalies, vaginal septum, ovaries |
| T2w TSE | Uterus (long-axis view) | Uterine anomalies |
| T1w SE (optional) | Same as T2w TSE (axial or sagittal) | Ovaries: characterization, hemorrhage |
| T2w with FS (optional) | True pelvis (axial) | Ectopic endometrium |

Fig. 12.23a, b Absence of the uterus and vagina on sagittal (a) and axial (b) T2w SE images.
Fig. 12.24a, b Uterus didelphys. Axial T2w SE images. a Good visualization of the two parallel cervical canals. b Demonstration of two vaginas (arrows).

Fig. 12.25 Unicornuate uterus with noncommunicating left horn. Endometrium from the noncommunicating horn can enter the peritoneal cavity through the uterine tube and cause endometriosis.

Fig. 12.26 Uterus bicornis unicollis. T2w TSE image.
Unicornuate Uterus
A unicornuate uterus is the result of incomplete or absent development of one of the müllerian ducts. The former is characterized by the presence of a rudimentary horn with or without functioning endometrium. Clinically, a unicornuate uterus is associated with a very high rate of miscarriage and complications during delivery. A noncommunicating horn containing functioning endometrial tissue can cause endometriosis (Fig. 12.25). A unicornuate uterus appears banana-shaped and tends to be deviated to one side. The typical triangular appearance of the uterine body is absent, but the zonal anatomy is preserved. If a rudimentary horn is present, it is similar in signal intensity to myometrium and may be distended by blood if it has an endometrial cavity.27,28 Women with this anomaly should be evaluated for the presence of ectopic endometrial tissue outside the uterus.
Bicornuate Uterus
A bicornuate uterus results from partial nonfusion of the müllerian ducts. The septum separating the two horns is composed of myometrium and may extend to the internal os (bicornis unicollis) or the external os (bicornis bicollis) (Figs. 12.26, 12.27).
Fig. 12.27a, b Uterus bicornis bicollis. Axial T2w TSE images. a The typical zonal anatomy (endometrium, junctional zone, myometrium) allows good identification of the two uterine horns (arrows). b Parallel arrangement of the two cervical canals.
MRI will demonstrate two uterine cavities lined by normal endometrium. The best image quality is again achieved during the secretory phase. The fundus (or roof of the uterus) is concave (with a depression of > 1 cm) and the intercornual distance is increased (> 4 cm).28,29 Fundal concavity is best appreciated on oblique coronal T2w images angled to the long axis. The septum has the same signal intensity as endometrium on all pulse sequences. The lower portion of the septum may be composed of connective tissue, reflected by lower T1w and T2w signal intensities. A cervical septum, if present, also has connective tissue signal intensity. Arcuate uterus is the mildest form of bicornuate uterus. It is characterized by a flattened fundus and a short septum more than 1 cm long.
Septate Uterus
A septate uterus results from incomplete resorption of the fibrous septum between the two horns. The septum may be short or extend down to the external os. Differentiation of a septate uterus from a bicornuate uterus is important for treatment planning because a septum can be removed endoscopically, while correction of a bicornuate uterus requires open surgery. These two anomalies are best distinguished on coronal T2w images angled to the uterine axis. Septate uterus differs from bicornuate uterus in that the distance between the two horns is smaller (< 4 cm) and the fundus is convex or slightly flattened (Fig. 12.28).29

Fig. 12.28 Septate uterus. T2w SE image.
Acquired Benign Uterine Disorders
Leiomyomas
Leiomyomas or fibroids are the most common tumors of the uterus, occurring in 30–40% of women during their reproductive years. They are composed of smooth muscle tissue with variable amounts of connective tissue and can occur as single or multiple tumors. Most leiomyomas arise in the body of the uterus (90%) and 5% in the cervix. An occasional fibroid is found outside the uterus. They may be submucosal, intramural, or subserosal. The majority of leiomyomas are intramural and are typically detected incidentally. However, depending on their size and location in the uterus, fibroids can block a uterine tube and cause infertility or obstruct delivery. Another 5–10% are submucosal or protrude into the endometrial cavity. They most often cause clinical symptoms (e. g., hypermenorrhea). Subserosal leiomyomas occasionally mimic a solid ovarian mass. Very rarely, torsion of a pedunculated fibroid can cause an acute abdomen. Other common clinical symptoms are enlargement of the uterus, menorrhagia, infertility, frequent miscarriage, and delivery complications.
| Sequence | Scan area/FOV (plane) | Comment |
| T2w SE | Uterus (axial and sagittal) | Determination of number, size, and site of fibroids; differential diagnosis: fibroid versus adenomyosis; characterization |

Fig. 12.29 Intramural leiomyomas. Sagittal T2w TSE image reveals two low-SI tumors in the myometrium. The larger tumor (arrow) in the posterior uterine wall shows degenerative changes. The smaller tumor is located in the anterior wall.

Fig. 12.30 Small intramural lyomyoma in the area of the left tubal ostium on coronal T2w SE image.
Appropriate treatment depends on the size and location of the fibroids as well as the severity of symptoms. In women who wish to have children, small submucosal leiomyomas can be removed by hysteroscopy, while intramural tumors are an indication for myomectomy. A more recent therapeutic option is uterine artery embolization (UAE), which is suitable for treating larger and multiple leiomyomas and can spare women from hysterectomy.30,31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree