CHAPTER 18 Vascular access placement and foreign body retrieval
The demand for long-term central venous catheters (CVCs) has increased dramatically over the past several decades. CVCs are used for infusion of drugs (e.g., antibiotics, chemotherapeutic agents), administration of blood products, blood sampling, hyperalimentation, and dialysis and apheresis. In the past, CVCs were placed exclusively by surgeons in the operating room. Interventional radiologists have now assumed the major role in the insertion of these devices.1 In some hospitals, vascular access placement is the most commonly performed procedure in the interventional radiology suite.
Image-guided placement of a CVC has several advantages over purely operative insertion.2,3 The risk of immediate complications such as pneumothorax, arterial puncture, and catheter malposition is almost completely eliminated. Precise positioning of the catheter tip is almost guaranteed. Finally, imaging is critical when placing catheters through occluded thoracic or neck veins or outside the chest (e.g., into the inferior vena cava [IVC]).
Access planning
Devices
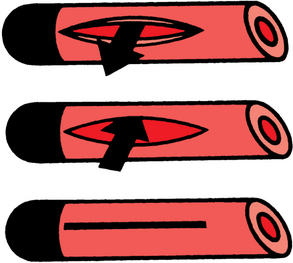
Tunneled, cuffed external infusion catheters are made of silicone or polyurethane-based material. They are manufactured with one, two, or three lumens in diameters from 3 to 12.5 French (Fr) (Fig. 18-1). These devices are inserted into a large vein and then travel through a subcutaneous tunnel before exiting the skin. A Dacron cuff embedded on the shaft incites a fibrotic reaction that ultimately secures the catheter in place and impedes the spread of infection from the skin exit site to the circulation. Infusion catheters have endholes or side valves (e.g., Groshong type). The valve design is intended to allow fluid injection and (possibly) blood aspiration but prevent blood or air from entering the catheter when it is not in use (Fig. 18-2). In theory, the risks of catheter occlusion and air embolism are reduced. Although valved catheters require less frequent flushing, they actually confer no benefit in terms of catheter dysfunction or vascular thrombosis.4–6 Some CVCs have a silver ion–impregnated cuff or silver coating that is meant to serve as an instant antimicrobial barrier; however, the value of this feature has been challenged.7,8 On the other hand, metaanalysis of existing literature supports the value of anti-infective agents incorporated into the inside and outside of the catheter body in reducing device-related bloodstream infections (BSI).9
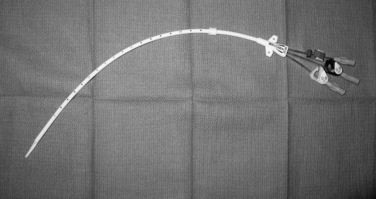
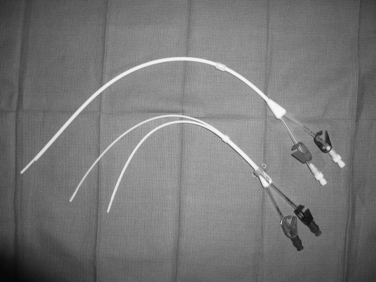
Tunneled hemodialysis and apheresis catheters are short, large-bore (typically 13.5 to 14.5 Fr), dual-lumen devices capable of handling high flow rates (e.g., 300 to 500 mL/min) (Fig. 18-3). These catheters have staggered or nonstaggered endholes or split lumens and are manufactured in preset lengths. From the right internal jugular (IJ) vein, 19- to 23-cm tip-to-cuff devices are usually appropriate. From the left IJ, 23- to 28-cm devices are often necessary. Tunneled hemodialysis catheters must be distinguished from nontunneled temporary catheters (usually placed by nephrologists) intended for several sessions of acute, emergent dialysis. No single catheter type has been proven to be most effective. Still, there is some evidence that split and symmetric tip catheters (e.g., Palindrome type) have a longer functional lifespan than the traditional staggered tip devices.10–14 In principle, separation of the uptake and return lumens is necessary to prevent recirculation, which can significantly diminish the efficiency of dialysis.15 Even so, the symmetrically tipped Palindrome catheter is reported to achieve the lowest recirculation rates among the various catheter models that are available.14
Implantable ports are constructed from a variety of materials, including titanium and plastic (Fig. 18-4). A silicone or polyurethane catheter is connected to a reservoir with a silicon window that is accessed through the skin using a noncoring (Huber) needle. The entire system is buried in the subcutaneous tissue of the chest or arm (or even the thigh or abdomen). These devices are available in single- and double-lumen configurations. Ports provide central venous access without the need for an external catheter. Small ports are designed for children, patients with minimal subcutaneous fat, or arm placement. So-called power ports are suitable for rapid intravenous (IV) contrast injection during computed tomography (CT) examinations. It is purported that the overall frequency of device and BSI is lower with ports than external tunneled CVCs, but not every study has confirmed this tenet.16,17
Peripherally inserted central catheters (PICCs) are essentially long IV lines placed from a peripheral arm vein (or occasionally the IJ) into the central venous system (Fig. 18-5). Single-, double-, and triple-lumen configurations are available in sizes from 3.0 to 7.0 Fr. Some of the designs (Power type) are capable of handling the high flow rates demanded with injection of contrast material for CT scanning.
Device selection
The selection of the most appropriate catheter in an individual case should be made jointly by the referring physician, interventionalist, and patient. Several factors are considered, including intended purpose, frequency and duration of use, underlying risk factors for infection or venous thrombosis, and patient preference for an external or implanted device (Table 18-1). Tunneled infusion catheters and implantable ports are only appropriate when the anticipated duration of need for continuous or periodic vascular access is more than several weeks. Whereas the risk of device-related infections is probably lower with chest ports, the consequences of infectious and other port site complications are more problematic. Finally, it is wise for the operator to personally confirm the device request just before placement to avoid an egregious implantation error due to miscommunication or an acute change in patient’s condition.
Table 18-1 Guidelines for Access Device Selection
| Device | Purpose or Situation |
|---|---|
| External, tunneled catheter | Continuous use |
| Multiple, simultaneous uses | |
| Patient preference | |
| Implantable port | Intermittent use |
| Immunocompromised patient | |
| Patient preference | |
| High-flow tunneled catheter | Hemodialysis |
| Apheresis | |
| PICC | Short-term use (<2–3 mo) |
| Infrequent blood drawing | |
| (lifetime risk for dialysis is low) |
PICC, peripherally inserted central catheter.
Venous access
Access route
CVCs are usually inserted through the IJV (tunneled catheters and ports) or upper arm vein (PICCs). The right IJV is preferred over the left because long-term catheter function is better and device-induced venous disease is less common.4 The subclavian vein should be avoided if at all possible; symptomatic or asymptomatic stenosis or thrombosis is much more likely to occur with this route.18–20 It is standard teaching to avoid placement on the side of the body that has undergone (or will undergo) mastectomy, radiation therapy, or axillary lymph node dissection, which may compromise lymphatic or venous limb drainage or wound healing.
If an occluded central vein can be recanalized or simply traversed with a guidewire (from the neck or the groin), catheter insertion still may be feasible.21 Some practitioners will attempt to place a vascular access device into an external jugular vein or enlarged collateral veins (e.g., intercostal vein).21,22 If no central thoracic vein is available, most operators will then turn to the common femoral vein. Practically, this situation occurs in patients requiring long-term hemodialysis. However, thrombotic, stenotic, and infectious complications are greatest with tunneled femoral catheters, which are prone to dysfunction and retraction 23-25 As a last resort, central venous access is obtained directly into the IVC through a translumbar or transhepatic approach.26–28
Patient preparation
With the notable exception of PICCs, vascular access insertion in an interventional suite demands the same aseptic environment as is found in an operating room. All procedures begin with antibacterial cleaning of surfaces that may come in contact with the patient, wide surgical preparation of the operating field, surgical scrub by all operators, and maintenance of strict sterile technique during the case. Some interventionalists give prophylactic antibiotics (e.g., 1 g of cephazolin IV) within 1 hour of placement of tunneled catheters or ports, even though the benefit of this step is questionable. Bacteremia or sepsis is a contraindication to placement of tunneled catheters and implantable ports.29 Coagulation parameters should be checked and corrected before the procedure (see Chapter 2). One recent study suggested that the standard coagulation thresholds for vascular interventional procedures may be relaxed with tunneled CVC placement (e.g., acceptable with platelet count greater than 25,000/mm3 or international normalized ratio less than 2.0).30
Venous entry (videos 18-1 and 18-2)
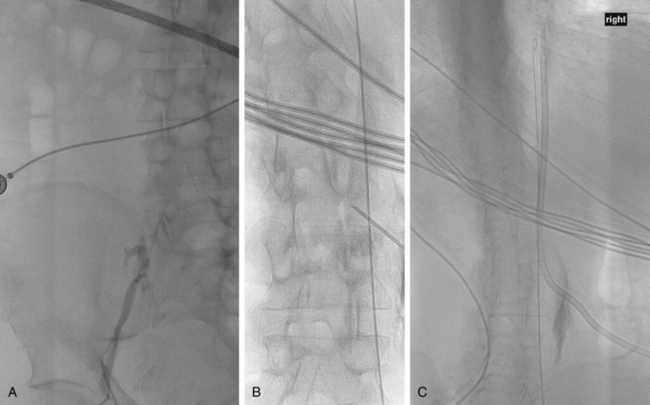
Doppler ultrasound spectral analysis of the IJs normally shows a triphasic waveform with respiratory variation. Dampening of the waveform suggests a central obstruction that may complicate or preclude device insertion. Sonographic guidance into the IJ is mandatory; this step unequivocally reduces the risk of inadvertent arterial puncture or pneumothorax and the number of needle passes required for venous entry.31 A site on the neck just above the clavicle is selected. The vessel is visualized with the transducer in a transverse plane while the needle is advanced from the side or from above (Fig. 18-6). The transverse orientation ensures that the carotid artery is constantly observed during needle insertion. Skin entry lateral to vein puncture results in a more favorable subcutaneous catheter pathway from the chest to the vessel. One group of investigators described a method for single puncture entry into the IJ directly from the intended catheter exit site using a curved needle.32
Some interventionalists prefer to access the vein with a 21-gauge micropuncture needle, 0.018-inch guidewire, and transitional 4- or 5-Fr dilator. The needle is advanced through the skin with real-time sonographic guidance. Weak blood return signals luminal entry. Sometimes, aspiration is required to confirm an intravascular location. Alternatively, continuous suction is applied to a saline-filled syringe and tubing connected to the needle until blood returns. If the needle coapts and then punctures the front and back walls of the vein, blood return occurs with needle withdrawal. The microwire is advanced down the jugular vein into the superior vena cava (SVC). Changes in needle angle or patient respiratory phase may be needed to redirect the wire if it passes up the jugular vein or into the subclavian or brachiocephalic vein. After the transitional dilator is placed, a standard guidewire is advanced into the right atrium or (preferably) IVC. If there is resistance to guidewire passage, contrast injection may show a central venous obstruction.
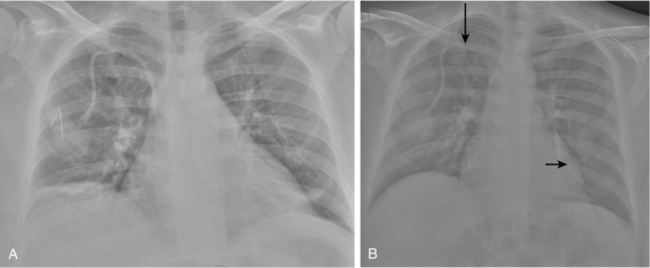
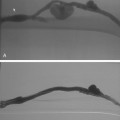
In the rare situation when a subclavian vein approach is necessary, the axillary vein is punctured lateral to the ribs in the subcoracoid region. This entry site almost completely eliminates the possibility of pneumothorax. It also ensures that the catheter is well within the subclavian vein as it passes through the costoclavicular space. If the catheter is extravascular at that site, chronic compression may occur and lead to catheter erosion and fracture (“pinch-off” syndrome)33,34 (Fig. 18-7). The axillary vein lies just inferior to the axillary artery (Fig. 18-8). The ultrasound transducer is oriented parallel to the vessel while the needle is advanced with real-time imaging.
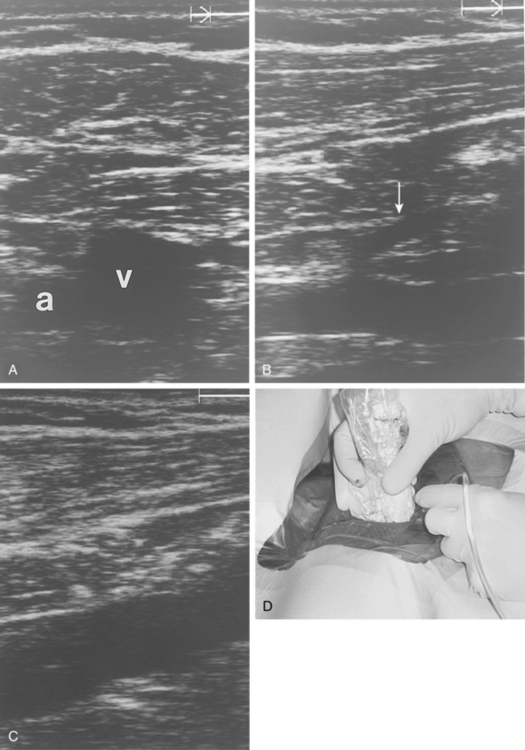
Figure 18-8 Axillary vein entry. A, Sagittal sonogram shows the relationship between the artery (a) and vein (v).B, Longitudinal image of the axillary-subclavian artery with a side branch (arrow).C, Longitudinal image of the axillary vein. D, Micropuncture needle is inserted under sonographic guidance with constant aspiration on the saline-filled tubing and syringe.
For common femoral vein insertion, sonographic guidance is most helpful although many practitioners still use the laterally located femoral artery pulse as a landmark. Catheters are tunneled laterally onto the thigh or lower abdomen (see later discussion).
IVC puncture may be required if all potential access sites in the chest, arms, and groin are exhausted.22,27,28,35 Prior CT scans are reviewed to identify any obstacles or contraindications to the intended route. Initially, a sheath, guidewire, or catheter may be placed into the IVC from the groin, although this step is not mandatory (Fig. 18-9). Bony landmarks alone may be used for caval entry. The patient is then placed prone on the interventional table. A skin site is chosen on the right flank above the iliac crest just lateral to the vertebral column. It is critical to plan a cephalic track into the IVC to avoid a sharp right angle turn as the needle enters the vessel, which can make sheath and catheter insertion exceedingly difficult. A long, 18- to 20-gauge needle is then advanced toward the catheter/wire in the IVC or toward the right lateral aspect of the spine in the calculated position of the IVC from prior imaging studies. Biplane fluoroscopy or frequent tube angulation is necessary to avoid overshooting the vena cava. Once blood is aspirated and contrast injection confirms the location, the remainder of the procedure is similar to catheter placement from other access routes. A stiff guidewire is necessary for placement of the peel-away sheath.
Transhepatic venous access is necessary for individuals whose only patent central vein is the suprarenal IVC. Even though this route is feasible in most cases, long-term catheter function is a serious problem.26,28 Finally, transrenal venous entry into the IVC has been described for patients with end-stage renal disease who require tunneled dialysis catheters.36
Catheter tip position (video 18-3)
The manufacturers of most vascular access devices instruct the operator to place the catheter tip in the SVC or at the cavoatrial junction. However, the long-term failure rate of CVCs placed with the tip at these locations at the termination of the procedure is significant. Most CVCs migrate 3 to 4 cm upward from the supine to standing position; PICCs move about 2 cm caudally when the arm is adducted after placement.37–39 Although not widely understood nor even accepted outside the interventional radiology community, it is now standard of practice to position the catheter tip in the upper to mid-right atrium with the patient supine.40,41 At this location, the catheter endholes will rarely become obstructed by a fibrin sheath because the tip hangs freely in the right atrium rather than plastered against the caval wall (Fig. 18-10). Several nonrandomized clinical studies lend credence to this approach.4,42–45 Because all of these devices are composed of soft polyurethane or silicone material, atrial dysrhythmias are quite rare (and easily managed by slight catheter withdrawal) and cardiac perforation is essentially reportable46–48 (see later discussion).
Device placement
Hemodialysis and apheresis catheters
Up to 50% of chronic hemodialysis patients develop vein stenoses or frank occlusions after placement of temporary or tunneled venous catheters through the subclavian or axillary vein route.18–20,49 In addition to causing disabling symptoms, these obstructions can interfere with the construction of future hemodialysis access. For this reason, CVC in patients on chronic hemodialysis must be placed in the right (or left) IJ whenever possible.
From an accessible site on the chest wall, the catheter is tunneled over the clavicle. Generous dissection of the subcutaneous tissues at the venotomy site and a somewhat low IJ puncture can prevent kinking of the catheter as it makes a turn out of the vein and toward the chest. For catheters with staggered lumens, the arterial lumen (proximal port) is positioned with the endhole away from the right atrial wall for optimal function. The catheter tip should be readjusted if rapid aspiration of blood is not possible after placement. Some dialysis units prefer to fill catheters with high-dose heparin (1000 or 5000 units/mL) to prevent thrombosis. Others opt for citrate solution to avoid the possibility of inadvertent injection of the anticoagulant.
Implantable ports (video 18-5)
Ports may be placed in the chest or arm (or even the thigh), depending on the patient’s lifestyle and preference.50–54 Chest ports are easiest to access. The intended port site must have enough subcutaneous fat to accommodate the device easily and avoid wound dehiscence or skin breakdown. Of note, port incision dehiscence is also a concern in patients who have received bevacizumab therapy within 10 days of insertion.55 However, a port can be difficult to access if there is too much surrounding adipose tissue or if it is poorly supported by underlying bone. Ideally, the port septum should be about 0.5 to 2 cm below the skin surface. The operator should avoid placement in breast tissue or the axilla.
After venous access is obtained (see earlier discussion), a 3- to 5-cm transverse incision is made on the anterior chest wall using a No. 10 or 15 blade after infiltration with 1% or 2% lidocaine with epinephrine. Bleeding from small arterial branches always stops with manual compression or brief occlusion with a small hemostat. A pocket is created inferior to the incision using sharp and blunt dissection. The pocket should be large enough to accommodate the port and allow easy closure of the wound. Some interventionalists (but not most) place two stay sutures from eyelets in the port base through the anterior fascial plane to secure it in place after insertion into the pocket.54
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree