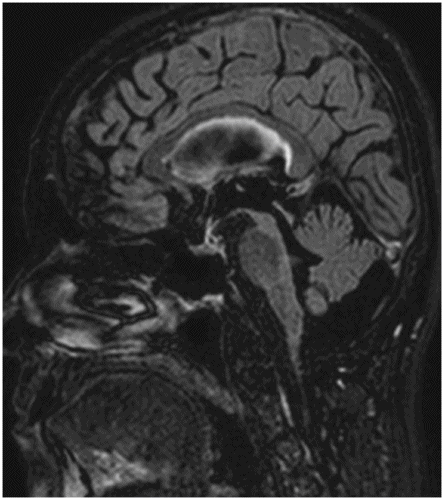
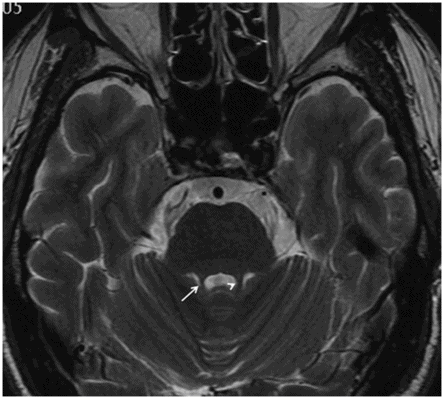
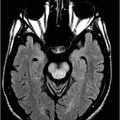
Sagittal T1WI through the midline.
Progressive Supranuclear Palsy
Primary Diagnosis
Progressive supranuclear palsy
Differential Diagnoses
Frontotemporal dementia (FTD)
Corticobasal syndrome (CBS)
Parkinson disease (PD)
Parkinson-plus syndrome (PPS)
Imaging Findings
Fig. 11.1: Sagittal T1WI. Fig. 11.2: Sagittal FLAIR image through the midline demonstrates foreshortening and significant atrophy of the midbrain, with relative sparing of the pons (known as humming bird sign or penguin-silhouette sign). Also, note that the superior border of the midbrain has slight concavity instead of regular convexity (due to significant atrophy). Fig. 11.3: Axial T2WI sequence through the midbrain demonstrates decreased anteroposterior diameter at the level of the superior colliculi (< 12 mm), giving the midbrain a Mickey Mouse appearance. Also, the loss of lateral convexity of the cerebral peduncles (morning glory sign) is noted. Fig. 11.4: Axial T2WI sequence through the pons demonstrates bilateral superior cerebellar peduncle atrophy that is more severe on the right side (arrow) compared to the left side (arrowhead).
Discussion
The gradually progressive supranuclear palsy (downward gaze palsy), Parkinsonism (rigidity, slow movements, and illegible handwriting), and frontotemporal-type dementia in a patient who is aged 40 years or more epitomizes the typical presentation of progressive supranuclear palsy (PSP). Imaging studies demonstrating characteristic midbrain atrophy further confirm a diagnosis of PSP.
The complex and extremely heterogeneous group of diseases including FTD, CBS, PD, and PPS overlap and share multiple clinical features. Thus, on a clinical basis, it is extremely difficult to differentiate between these disease types. It is also difficult to differentiate between these diseases immunopathologically, as they all can be tau-immunoreactive. Thus, all of these diseases are considered variants on a spectrum of a common histopathologic process. Individual disease types can be diagnosed by combination of clinical and radiologic findings.
Progressive supranuclear palsy is a tauopathy accompanied by a heterogeneous clinical syndrome and variable histopathologic findings. As expected in any tauopathy, PSP shares clinical findings with other tau-related neurodegenerative diseases and demonstrates symptoms related to degeneration of the cerebral cortex, deep gray matter nuclei of the basal ganglia, brainstem, and cerebellum. The clinical and pathologic spectrum of PSP depends upon the degree and region of brain involved. In one extreme, PSP can be associated with predominant cortical involvement and presents as PSP-progressive non-fluent aphasia (PSA-PNFA). However, classic PSP as described by Richardson (PSP-R), is at the other end of the spectrum that predominantly features degeneration of the brainstem nuclei. Other PSP variants, such as PSP-associated Parkinsonism (PSP-P), PSP-associated pure akinesia with gait freezing (PSP-PAGF), and PSP-corticobasal syndrome (PSP-CBS) are in the middle of the spectrum.
Clinical presentations of typical PSP-R include Parkinsonism features (rigidity and bradykinesia), supranuclear gaze palsy, and postural instability with falls. Characteristic impairment of downward gaze results in problems reading, eating, and walking. Nystagmus is also common. Varying degrees of associated neuropsychiatric disturbances such as cognitive decline, psychomotor slowing, impaired decision-making, and apathy are also common. Obsessive-compulsive and disinhibitory features have also been described in patients with PSP-R.
In patients with PSP, there is severe involvement of the subthalamic nucleus, pallidum, striatum, red nucleus, substantia nigra, pontine tegmentum, third nerve nuclei in the pons, cerebellar white matter, and dentate nuclei. In PSP-R, the substantia nigra pars compacta and ventro-tegmental areas are most severely affected, with selective destruction of the dopaminergic nigrostriatal system. This discriminate tissue damage explains why PSP-R is non-responsive to levodopa. Although the substantia nigra can be affected in other PSP variants, it is less severe compared to PSP-R.
Generalized brain atrophy is commonly seen in PSP, with predominant involvement of the frontal lobes. The distinguishing imaging finding in PSP is the striking atrophy of the midbrain in comparison to the rest of the brainstem. This distinctive feature has been described as a humming bird sign or penguin-silhouette sign on midline sagittal images and the Mickey Mouse and morning glory sign on axial images. Several quantitative measurements have been proposed for accurate diagnosis of PSP. The most specific and most sensitive sign in discriminating PSP from PD or other multiple system atrophy subtypes is Quattrone’s MR Parkinsonism index. It is calculated as the ratio of pons area to midbrain area measured in the midsagittal plane, multiplied by the ratio of the middle cerebellar peduncle and superior cerebellar peduncle width. On FDG-PET, there is hypometabolism in the frontosubcortical regions (anterior cingulate gyrus and lingual gyrus).
The histopathologic hallmarks of PSP are the presence of star-shaped argyrophilic astrocytic tufts and globose neurofibrillary tangles in neuronal cytoplasm that are tau-immunoreactive and have four repeats of the phosphate-binding domain (i.e., 4R tauopathy) similar to CBD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree