Hypertrophic Olivary Degeneration
Primary Diagnosis
Hypertrophic olivary degeneration
Differential Diagnoses
Medullary infarction
Medullary tumor
Demyelinating plaque
POLG or SURF1 mutation
Imaging Findings
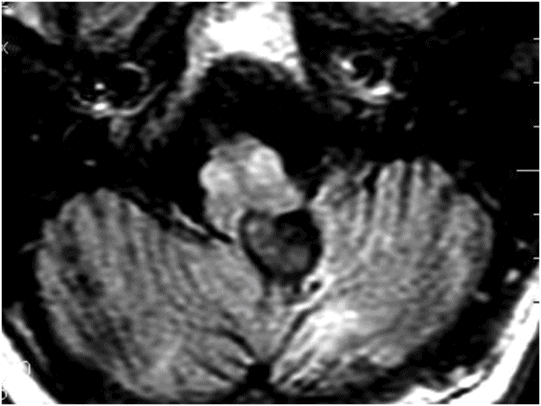
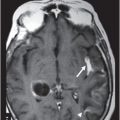
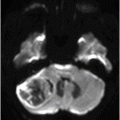
Fig. 132.1: Midline sagittal precontrast T1WI demonstrated a heterogeneous lesion with mixed signal intensity in the dorsal aspect of the pons. Fig. 132.2: Axial T2WI through the pons demonstrated a predominantly hyperintense lesion, surrounded by a thin hypointense rim, secondary to hemosiderin deposition in the posterior aspect of the pons involving the expected area of central tegmental tract and the right superior cerebellar peduncle. Fig. 132.3: Axial FLAIR and Fig. 132.4: T2WI through the level of lower medulla demonstrated hyperintensity and mild enlargement of bilateral olives, with sparing of the adjacent other medullary structures.
Discussion
Presentation with palatal myoclonus is highly suggestive of pathology at the medulla, particularly with involvement of the inferior olivary nucleus (ION) at the olive. Bilateral olivary enlargement and hyperintensity on T2WI with sparing of the adjacent medullary structures is diagnostic of hypertrophic olivary degeneration (HOD), due to a venous vascular malformation at the dorsal pons, disrupting the superior cerebellar peduncle (SCP) decussation and the central tegmental tract. Acute onset of visual disturbances six months prior to presentation was likely due to hemorrhage in the venous vascular malformation.
Medullary demyelination and infarction typically accompany T2 hyperintensity that lacks ION enlargement. Tumor (astrocytoma or metastasis) may involve the medulla; however, the exclusive involvement of the olives, without involvement of surrounding brain tissue, is not suggestive of tumor. Bilateral HOD has been described with POLG and SURF1 mutation. In these variations of HOD, there are no structural lesions seen at the brainstem. Additional cystic degeneration of the bilateral cerebellar hemisphere is an additional imaging feature of POLG mutation, which is not seen in this patient.
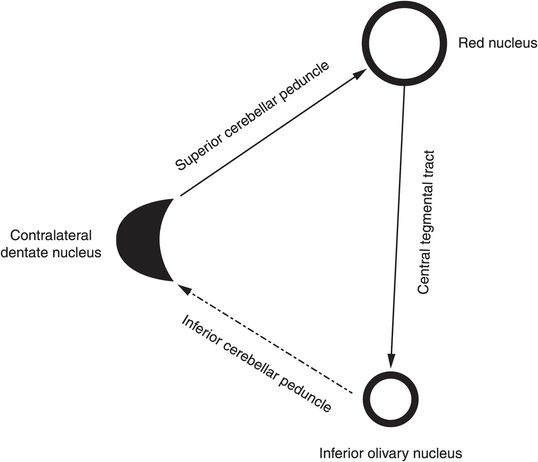
Hypertrophic olivary degeneration, first reported in 1887 by Oppenheim, is a distinct and rare form of transsynaptic degeneration (neuronal loss due to loss of input) that occurs as a result of lesional growth in the dentato-rubro-olivary pathway (Guillain-Mollaret triangle). This extrapyramidal system is formed by the ipsilateral ION in the medulla, the red nucleus (RN) in the midbrain, and the contralateral dentate nucleus (DN) in the cerebellum.
Dentatorubral fibers connect the RN with the contralateral DN via the SCP that crosses the midline at the decussation of the SCP at the inferior midbrain/superior pons. The RN and the ipsilateral ION are connected via the central tegmental tract. Fibers from the ION project to the contralateral cerebellar cortex through the olivocerebellar tract via the inferior cerebellar peduncle (ICP) and then to the DN (see Fig. 132.5).
Lesions disrupting the central tegmental tract cause ipsilateral HOD, whereas lesion growth at the DN or SCP causes contralateral HOD. Bilateral HOD is seen when a lesion involves both the central tegmental tract and the SCP. As there is no direct white matter connection between the ION and the contralateral DN, disruption of this limb of the Guillain-Mollaret triangle does not cause HOD. Common lesions that are involved in HOD include venous vascular malformation (as in this patient), cavernoma, demyelination, infarction, tumor, or iatrogenic-based lesions. Deafferentation of ION causes supersensitivity denervation and HOD. Histologically, this entity is characterized by neuronal degeneration, vacuolation and swelling, gliosis, and tissue demyelination in the ION. In some cases, no cause could be found and these are termed idiopathic HOD.
Pathologic changes in HOD have been described in six stages. In the first stage, no significant changes are noticed immediately after the procursive insult. The second stage begins approximately three weeks after the initial insult and is distinguished by amiculum degeneration, and consequently, notable olivary neuronal hypertrophy. During the third stage, glial hypertrophy occurs that ushers in the fourth stage, subsequent olivary enlargement. In the fifth stage, pseudohypertrophy develops as neurons degenerate; however, large gemistocytic astrocytes persist, denoting the fifth stage of HOD. Finally, more than a year after the initial injury, during the sixth stage, the ION undergoes atrophy. Although hypertrophy resolves, T2 hyperintensity rarely normalizes.
Hypertrophic olivary degeneration affects children and adults; the majority of patients present with unilateral HOD. Clinical findings associated with HOD include palatal tremor (PT; 1–3 Hz rhythmic involuntary movement of the oropharynx), ocular myoclonus, and dentatorubral tremor of the upper extremity. Palatal tremor can be an essential diagnostic clue when no etiologies can be identified. Occurrence of symptomatic PT tends to correlate with onset of changes in the ION, reaching its maximum soon after ION dysmyelination peaks. Deafferentation of the ION causes disinhibition of the nucleus, triggering spontaneous rhythmic firing, and stimulating the contralateral dentate to lower motor neuron projections; therefore, ION degeneration causes symptomatic PT. Alternatively, the disrupted dentato-rubro-olivary pathway may become unable to inhibit cranial nerve motor nuclei firing.
On MR, T2-weighted images are best in depicting the abnormal and affected ION, which is typically increased in size and shows a hyperintense signal. Imaging findings correlate well with the pathologic features. Increased T2 signal is usually seen one month after a cerebellar and/or brainstem infarct. Olivary enlargement is noted as early as six months after injury. Inferior olivary nucleus expansion may cease in 10–16 months; however, T2 signal abnormalities may persist for years or even indefinitely. With diffusion tensor imaging, it has been shown that there is decreased fractional anisotropy of the involved fibers and that the disrupted tracts are smaller compared to the uninvolved side. The myoclonus associated with HOD has been successfully treated with benzodiazepines, carbamazepine, or 5-hydroxytryptophan.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree