11 Breast Anatomy
Introduction
Understanding breast anatomy is important to recognizing the disease processes that may occur within the breast. Mammary glands are unique to mammals and are unlike any other anatomical part of the body. They are essentially modified exocrine glands responsible for lactation, the production of milk. Being knowledgeable about the embryological development and anatomy of the breast is crucial for radiologists to determine radiological–pathological concordance or discordance after a breast biopsy, to understand imaging correlates, and to plan interventional procedures. The historical understanding of breast anatomy is based on Sir Astley Cooper’s book which was published in 1840, but significant advances in medicine and imaging have improved our overall understanding of breast anatomy.
Embryology and Development
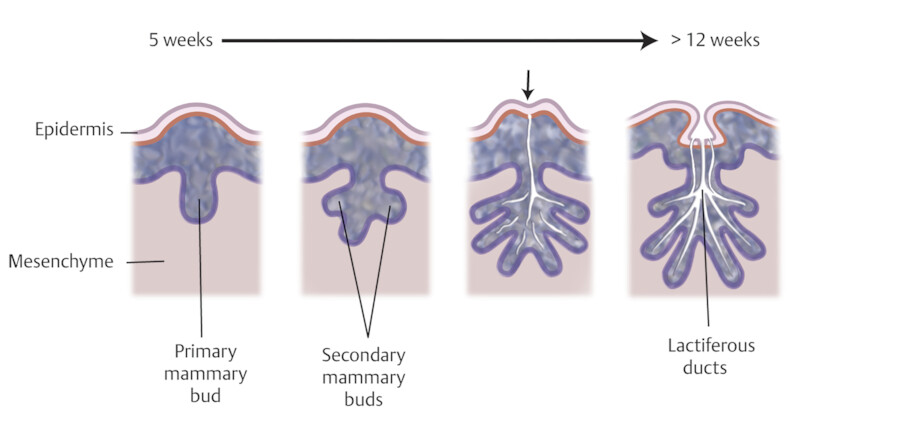
The breasts develop from an ectodermal milk line running from the axilla to the medial thigh on each side. 1 These paired mammary ridges develop on the ventral surface of the embryo and much of the line atrophies except at the pectoral location. 1 , 2 The breasts start to develop during the fourth to sixth weeks of embryonic life from the ectoderm, which are skin precursor cells. 3 Under influences of maternal hormones and genetics, the mammary ridges thicken and form the human breasts at the level of the fourth intercostal space bilaterally. Mammary buds are usually formed by the fifth week of gestation. Between the 5th and 12th weeks, the mammary buds continue to grow and form secondary buds and mammary lobules. After the 12th week, the secondary buds continue to develop and branch, and ultimately develop into ducts that extend from the nipple to the breast lobules 3 , 4 (Fig. 11‑1).
The nipple–areolar complex begins to develop between 12th and 16th weeks of gestation. The nipples are inverted at this time but then evert when the fetus is near full term or after birth when the sebaceous glands and erectile tissue of the nipple–areolar complex develop. During this time, the areola increases in pigmentation between 32nd and 40th weeks. 5
The nipple can fail to evert after birth which is often hereditary and a result of a hypoplastic ductal system. Incomplete regression of the milk line can result in an accessory nipple anywhere along this region. An accessory nipple (polythelia) and/or breast tissue (polymastia) most commonly develop in the axilla or inframammary fold but can occur anywhere along the milk line (Fig. 11‑2 , Fig. 11‑3). Polythelia can occur in both sexes with the incidence varying greatly in literature. One study reports an incidence of accessory nipples to be 2.5%. 5 , 6 , 7

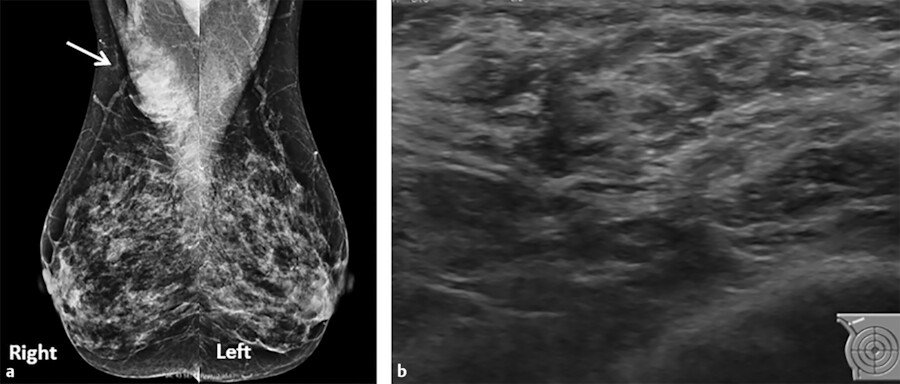
Other developmental abnormalities can include hypoplasia (underdevelopment of the breast), amastia (absence of one or both breasts), or athelia (absence of one or both nipples). 2 When amastia occurs unilaterally, it is associated with the absence of the pectoralis muscle, while amastia that occurs bilaterally is associated with other birth defects. 7 , 8 It is important for both clinicians and radiologists to be knowledgeable about the development and appearance of breast buds because the most common cause of amastia is iatrogenic resulting from biopsy of a developing breast bud and leading to a marked deformity later in life. 8
During this time of growth, the supporting structures and tissues of the breast are developing and maturing under the influence of maternal hormones. While the ducts and alveoli are made from the ectoderm, the connective tissue and vasculature of the breast are formed from mesenchyme. Shortly after birth, when the maternal hormones are no longer present, there is a pause in breast growth and does not restart again until puberty. The developmental period of the breast bud and nipple is the same process for males and females.
When puberty starts, there is a difference in the development of male and female breast tissue. In the male breast during puberty, the ducts and stromal tissues involute and atrophy. Therefore, the male breast is mostly comprised of fat. On the other hand, in the female breast, the estrogenic effects stimulate the growth of the supporting soft tissue structures as well as the ducts and lobules. During puberty in females, the breast mound increases. The subsequent enlargement and outward growth of the areola then result in a secondary mound. 1
Breast Parenchyma
The adult breast lies between the second and sixth ribs and has a medial boundary being the sternal edge and a lateral boundary being the midaxillary line. The breast overlies the pectoralis major muscle superiorly, serratus muscle laterally, and upper abdominal oblique muscles inferiorly. 3 The breast is composed of both epithelial and stromal elements. About 10 to 15% of the breast volume is made of epithelial elements while the remainder of the breast is stromal elements. 9 The upper outer quadrant of the breast usually contains the most amount of glandular tissue.
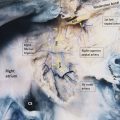
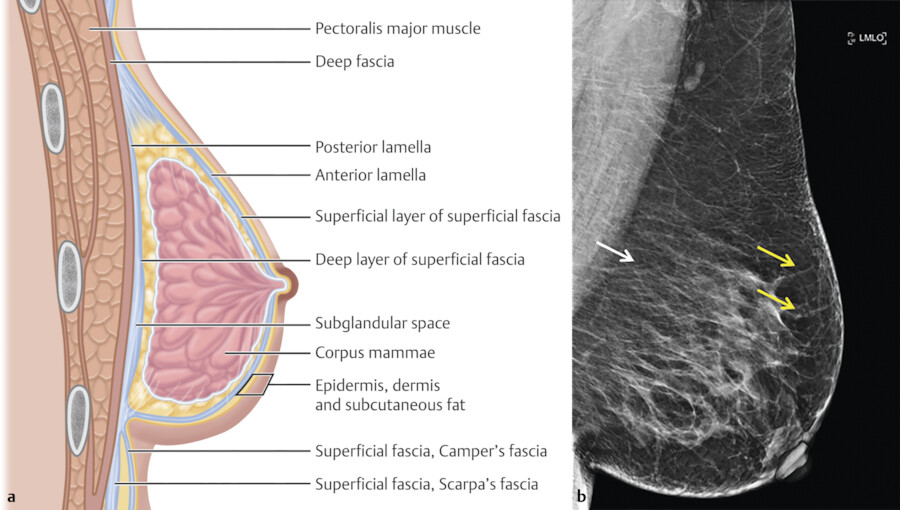
The skin of the breast is thin and contains hair follicles, sebaceous glands, and sweat glands. Two fascial layers are present within the breast. The superficial fascia lies deep to the dermis and the deep fascia lies anterior to the pectoralis major muscle fascia. 9 The superficial pectoral fascia envelops the breast and is continuous with the superficial abdominal fascia of Camper. The undersurface of the breast lies on the deep pectoral fascia which covers the pectoralis major and anterior serratus muscles. Connecting the two fascial layers are the Cooper suspensory ligaments which allow for support of the breast. The suspensory ligaments of Cooper are perpendicular to the orientation of the skin (Fig. 11‑4).
There is a retromammary bursa or space (Fig. 11‑4) which is filled with loose tissue, and along with the suspensory ligaments of Cooper, allows the breast to move freely against the thoracic wall. 9 The average breast measures 10 to 12 cm in diameter with an average thickness at the center of approximately 5 to 7 cm.
Ducts and Lobules
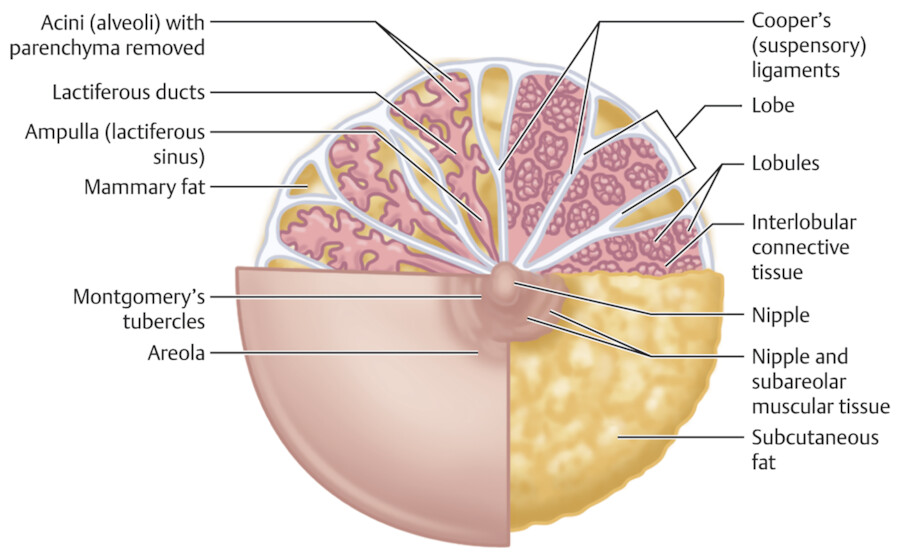
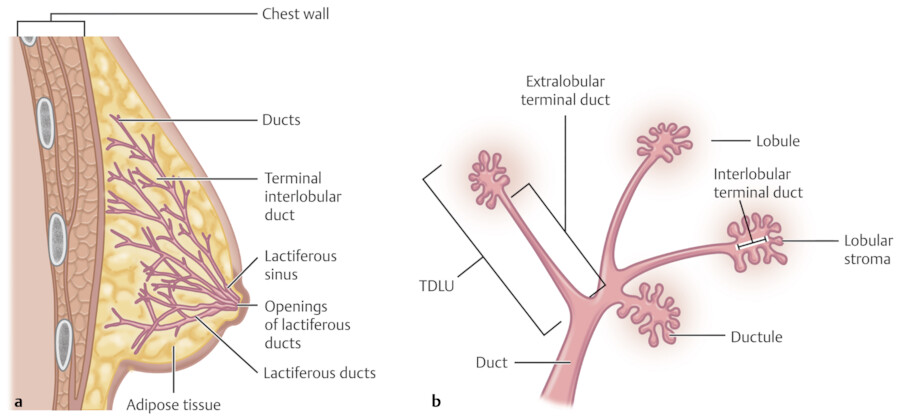
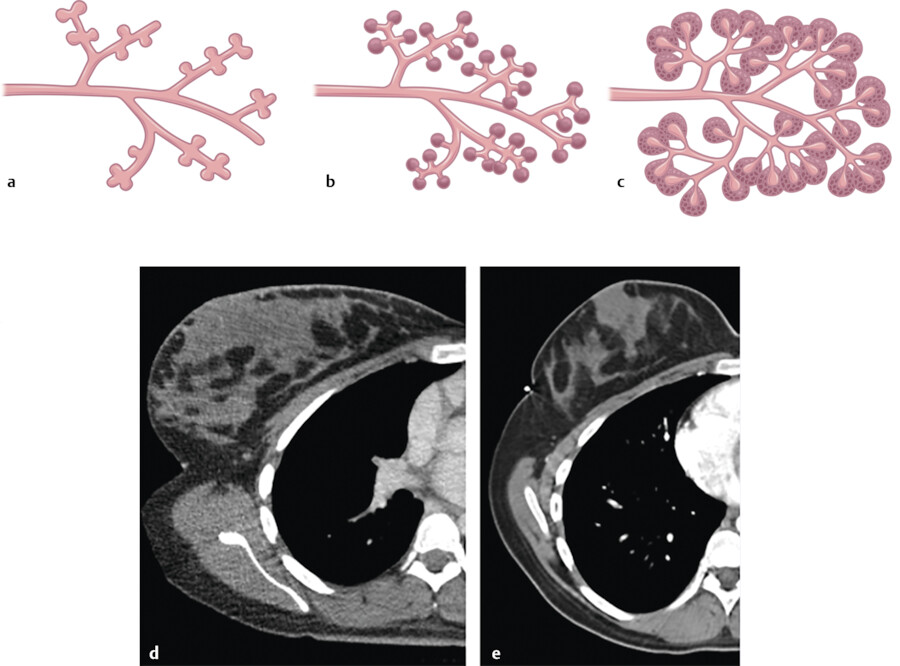
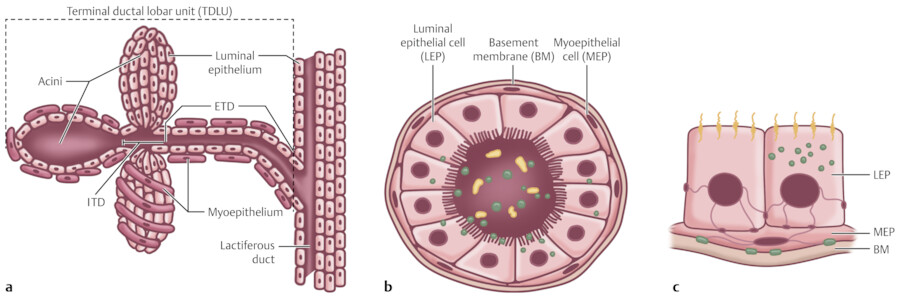
The breast is composed of approximately 15 to 20 lobes and these lobes are further divided into lobules. The lobules are made up of branched alveolar glands. Each lobe drains into a major lactiferous duct (Fig. 11‑5). The lactiferous ducts dilate into a lactiferous sinus beneath the areola and then open through a constricted orifice onto the nipple. The space between the lobes is filled by connective tissue including fat. During the development of the breasts at the time of puberty, the ducts grow and divide and form terminal end buds. The terminal end buds then form new branches and small ductules termed alveolar buds. The alveolar buds differentiate into the terminal structure of the resting breast called acines or ductules. 8 Alveolus sometimes refers to the end secretory unit at rest or nonpregnant state while acines refers to the fully developed unit in pregnancy or lactation. 8 , 10 Typically, there are hundreds of acinar cells within each breast. The terminal ductal–lobular unit (TDLU) refers to the basic functional unit of the breast with 30 to 50 acinar cells grouped into a lobule and the associated ducts. A normal TDLU ranges between 1 and 4 mm in size (Fig. 11‑5 , Fig. 11‑6 , Fig. 11‑7).
In the immature breast, the ducts and alveoli are lined by a two-layer epithelium that consists of a basal cuboidal layer and flattened surface layer. In the adult breast, the epithelium proliferates and becomes multilayered. The alveolar cell types have been described: superficial (luminal) A cells, basal (chief) B cells, and myoepithelial cells. 8 Myoepithelial cells are located surrounding the alveoli and are contractile units that are stimulated by hormones such as prolactin and oxytocin (Fig. 11‑8).
If pregnancy occurs, the breast lobules which are composed of epithelial lobular cells and underlying contractile myoepithelial cells differentiate for lactation. 3 With the presence of prolactin hormones, the lobules are stimulated to produce and secrete milk. In the first 4 weeks of pregnancy, increased levels of estrogen cause the ducts and lobules to form and branch. By the eighth week of pregnancy, the breasts are larger in size with associated increased vascularity and increasing pigmentation of the nipple–areolar complex. During the second trimester, the lobular formation is greater than that of the ductal sprouting due to increased levels of progesterone. The remainder of the pregnancy is associated with continued breast enlargement from alveoli being filled with colostrum, and hypertrophy of the myoepithelial cells and connective tissues of the breast 5 , 8 (Fig. 11‑7). If the pregnancy is interrupted early or if breast-feeding is stopped, these lobular cells then return to the nonfunctioning state (Fig. 11‑7).
During menopause and the aging process, both the ductal and lobular system of the breast start to atrophy and involute thus decreasing the density of the breast parenchyma. This results in a greater volume of the breast to be composed of fatty elements. This is the result of changes in the circulating hormones and a decrease in ovarian estrogen production. During menopause, ducts, lobules, and lymphatic channels all decrease in number. The ductal system will remain while the lobules shrink and collapse. 5 , 8
A wide spectrum of benign and malignant disease processes may occur within the breast that originate from the ducts, lobules, or connective tissues. The most common malignant diseases of the breast include ductal carcinoma in situ (DCIS), invasive ductal carcinoma, and invasive lobular carcinoma. There is evidence that supports the fact that both ductal carcinoma and lobular carcinoma arises in the TDLU. 11 DCIS is “noninvasive” or “preinvasive” ductal carcinoma because the basement membrane of the ducts is not involved by tumor cells. It typically presents on mammogram as suspicious linear or pleomorphic calcifications in a grouped, linear, or segmental distribution (Fig. 11‑9, Fig. 11‑10). Invasive cancers usually present as irregular solid masses. About one in five newly diagnosed breast cancers will be DCIS. Invasive or infiltrating ductal carcinomas comprise a majority of newly diagnosed breast cancers (up to 80%) while invasive lobular carcinomas are rarer (about 10%) (Fig. 11‑11).
The most common benign breast masses are cysts, fibroadenomas, and papillomas. Breast cysts are derived from the terminal duct lobular unit when there is blockage of the terminal acini with resultant dilation of ducts 12 , 13 (Fig. 11‑12). Fibroadenomas are the most common neoplasm of the breast and is thought to occur in 25% of asymptomatic women. 12 Fibroadenomas develop from the stroma and epithelial components from the breast lobule. Fibroadenomas usually present as a highly mobile, firm, nontender, and often palpable breast mass 12 , 14 (Fig. 11‑13).





Nipple–Areolar Complex
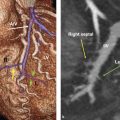
At full development of the breasts, the nipple–areolar complex most commonly is located at the fourth intercostal space. A mature breast usually is composed of 15 to 20 segments that converge at the nipple in a radial arrangement. The number of segments and the number of mammary ducts may vary. 15 , 16 , 17 There may be anywhere between 4 and 18 main mild ducts at the nipple, but the average is about 10 major collecting ducts that open at the nipple. 16 This ductal network can be very complex and heterogeneous and may not always follow a perfect radial pattern as commonly depicted. 3 The ducts are usually 2 mm in diameter but coalesce in the subareolar region into lactiferous sinuses which measure 5 to 8 mm in diameter 17 (Fig. 11‑6; Fig. 11‑14).
The nipple–areolar complex contains Montgomery glands which are embryologically between sweat glands and mammary glands and are capable of secreting milk. 15 The Montgomery glands open at the Morgagni tubercles, which are small raised papules on the areola 18 (Fig. 11‑5). The skin of the nipple is continuous with the ductal epithelium and therefore disease processes such as malignant neoplasms of the ducts may spread to involve the nipple. 15
Both nipple retraction and inversion may occur as a congenital or acquired process and may be unilateral and bilateral. Usually, bilateral and slow progressive nipple retraction is a benign process. 17 However, if a woman presents with new onset of unilateral nipple inversion, underlying malignancy or inflammation should be excluded by appropriate clinical and imaging work-up. 17 It is important that during mammographic imaging, the nipple is positioned in profile, because it may be mistaken for a mass. The nipple should be in profile for at least one mammographic view. On contrast-enhanced breast magnetic resonance imaging (MRI), the nipple has variable imaging appearances. Usually, the nipples are surrounded by a smooth thin rim of enhancement and are symmetric. 19 Sometimes the enhancement from an inverted nipple may be mistaken for a mass (Fig. 11‑15).


Benign and malignant pathological processes may involve the skin of the nipple. Benign skin diseases involving the nipple include eczema and psoriasis and are usually bilateral. If symptoms do not improve, a biopsy is necessary to exclude Paget’s disease of the nipple, a malignant condition involving the epidermis and usually associated with underlying DCIS and occasionally invasive ductal carcinoma. 20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree