15 Pulmonary Valve
Introduction
Knowledge of pulmonary valve and root anatomy is useful in understanding the spectrum of complicated conotruncal anomalies that arise from abnormal formation of the major vessels in this region. Despite the frequency of pulmonary valve diseases including congenital malformations, the pulmonary valve is the least studied valve by imaging. Along with the evolution of surgical techniques and introduction of new percutaneous procedures in recent years, imaging assessment of the pulmonary root and related pathologies has attracted more attention than before.
Pulmonary valve assessment is primarily dependent on echocardiography. However, because of their retrosternal location, the pulmonary valve and right ventricular outflow tract (RVOT) can be difficult to assess with transthoracic echocardiography especially in adolescents and adults. Furthermore, since the RVOT and pulmonary valve are anterior structures, transesophageal echocardiography is not the best tool for assessment of the pathology. With rapid advancement in imaging technology, cardiac computed tomography (CT) and magnetic resonance imaging (MRI) are being used increasingly for anatomical evaluation, functional assessment, and pathological diagnosis of the pulmonary valve. Postoperative evaluation of the pulmonary valve and outflow tract is among common MR referrals. MR is especially helpful in postoperative follow-up of artificial pulmonary valve function. Anatomical detail of the pulmonary valve and perivalvular structures can be optimally studied with CT scan.
The goal of this review is to offer a general perspective on the development of right outflow tract (OFT) and associated structures with a focus on the morphology and function of the pulmonary valve. Pathologies including congenital heart disease (CHD) are briefly discussed.
Embryology
The two fields of cardiac progenitors are now recognized as the primary, and secondary, or anterior, heart fields. 1 , 2 In mouse, there is firm evidence that the primary heart field gives rise to the left ventricle, with the secondary field forming both the right ventricle (RV) and the OFT. 1 Development of the semilunar valves occurs simultaneously with completion of the secondary (anterior) heart field.
The primordial outflow tract extends proximally from the distal ventricular groove to the pericardial reflections and demonstrates a characteristic dog-leg which divides it into two myocardial subsegments, a proximal subsegment or the conus (infundibulum) and a distal subsegment or the truncus. 1 , 3 The truncus arteriosus is a short segment interposed between the conus and the aortic sac (Fig. 15‑1). With further development, the aortic sac transforms into the extrapericardial ascending aorta and pulmonary trunks, and the truncus is remodeled into the intrapericardial portions of the aorta and pulmonary trunk. The boundary between the two parts of the primordial OFT becomes the sinotubular junctions. The primordial OFT is mainly myocardial. Gradual disappearance of the myocardium by apoptosis, transdifferentiation initially involves the truncus wall and the tissue surrounding the developing arterial sinuses. Later, further absorption of the left conus myocardium produces fibrous continuity between the leaflets of the aortic and mitral valves. On the right, conus myocardium remains as subpulmonary infundibulum. With extension of the developing fibroadipose tissue plane that already separates the aortic and pulmonary trunks, the muscularized partition becomes converted into the free-standing infundibulum of the pulmonary valve, and the supraventricular crest of the RV. As development proceeds, the single OFT undergoes remodeling into separate pulmonary and aortic arteries (Fig. 15‑1). This process involves interactions between diverse cell types, including myocardium, endocardium, and neural crest cells. Endocardial cells respond to signals from the overlying myocardium and undergo an epithelial-to-mesenchymal transformation to form the conotruncal cushions. Neural crest cells invade the extracellular matrix of the cushions and participate in aorticopulmonary septation. 4 OFT undergoes rotation during its remodeling. Rotation of the myocardium at the base of the OFT is probably essential to achieve normal positioning of the great arteries with respect to each other at the ventriculoarterial junction (VAJ). 5 , 6

The valves and their supporting sinuses are believed to develop from the conotruncal endocardial cushions around the distal part of the conus. 3 Valves are formed by the formation of cavities within the cushions. The central parts of the cushions form the leaflets and the peripheral parts arterialize to form the sinus walls. The improper fusion or dedifferentiation of the endocardial cushions is thought to be responsible for congenitally abnormal semilunar valves. The aorticopulmonary septation by the endocardial cushions is a complex process and involves interaction between diverse cell types, including myocardium, endocardium, and neural crest cells. 3 , 4 In addition to abnormal OFT septation caused by neural crest cell defects, a spectrum of conotruncal anomalies with abnormally positioned great arteries may arise from a perturbation of myocardial rotation including tetralogy of Fallot (TOF), persistent truncus arteriosus, double outlet right ventricle (DORV), and transposition of great arteries (TGA). 5 , 6
Anatomy
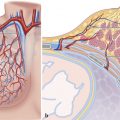
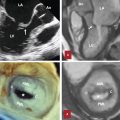
The pulmonary root is the part of the RVOT that supports the leaflets of the pulmonary valve. 7 , 8 It consists of three sinuses of Valsalva confined proximally by the semilunar attachments of the valvular leaflets and distally by the sinotubular junction. This relationship can change in CHD. Different nomenclature has been used to define the anatomical location of the pulmonary valve sinuses based on their spatial location in relation to the thorax or the heart itself 7 (Fig. 15‑2 , Fig. 15‑3). The pulmonary valve is in the left anterior of the aorta and forms an angle of approximately 30 degrees with the aortic trunk. In normal individuals, this angle is related to the length of the ascending aorta. In elongated tortuous ascending aorta, the angle between the two arteries will be increased (Fig. 15‑4). Because of the semilunar shape of the pulmonary leaflets (similar to the aortic valve) this valve does not have a ring-like annulus. The sinotubular junction separates the pulmonary valvular sinuses from the tubular component of the pulmonary trunk and demarcates the level of the zones of apposition (commissures) between the annuli (Fig. 15‑5 , Fig. 15‑6). Compared to the aortic root, the pulmonary sinotubular junction is less obvious on CT images. A second junction exists at the VAJ between the infundibular muscle and the fibroelastic arterial wall. The anatomical VAJ forms the annulus. The semilunar attachment of the valvular leaflets, which forms the hemodynamic VAJ, crosses the anatomic VAJ. The leaflets are thickened along their semilunar line of attachment. The fibrous interleaflet triangles are the areas of arterial wall proximal to the semilunar attachments of the leaflets, and therefore are incorporated within the ventricular cavity. The fibrous triangle tips point toward the commissures (Fig. 15‑6). The pulmonary valve is surrounded by the ventricular muscle. The musculature of the subpulmonary infundibulum raises the pulmonary valve above the ventricular septum to position the pulmonary valve as the most superiorly situated of the cardiac valves (Fig. 15‑7 , Fig. 15‑8). This anatomical feature makes possible the safe resection of the pulmonary valve, including its basal attachments within the infundibulum from the rest of the RVOT. 8 The length of the free-standing infundibulum varies and some cases may be too short to resect (Fig. 15‑7 d).







Arterial Supply
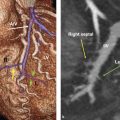
The conotruncal structures including the pulmonary valve are normally vascularized by anterior and posterior arterial branches from the right and left coronary arteries 9 (Fig. 15‑9). On the right side, the branches arise from the conal branch of the right coronary artery (RCA) or directly from the aorta. On the left side, they arise from the left anterior descending artery (LAD), the left main, or directly from the aorta. The right anterior conal branch is the most constant and conspicuous branch participating in the preconal circulation, also known as Vieussens’ arterial ring. 9 This collateral intercoronary connection extends between the conus artery and first right ventricular branch (left anterior conus branch) of the LAD artery. The Vieussens’ arterial ring will become dilated when there is proximal LAD artery occlusion or, less frequently, RCA occlusion 10 (Fig. 15‑10). Generally, three major collateral pathways at the conotruncal level provide circulation between the right and left coronary system in all congenital or acquired forms of one-sided coronary occlusion and are used as the basis for different classifications 10 (Fig. 15‑9). These three collateral circulation pathways include preconal (precardiac), retroconal (interarterial), and retroaortic.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree