17 Pleura, Chest Wall, and Diaphragm
On computed tomography (CT) scans, the pleura appears as a 1-to 2-mm-thick line between lung parenchyma and visualized ribs. Starting from the inside, this line represents the visceral and parietal pleura, extrapleural fat, endothoracic fascia, and innermost intercostal muscle. The latter is absent in the paravertebral region, resulting in a distinctly thinner lining. On axial scans, the most posterior ribs are always the lowest in the thoracic cage, with each more anterior rib arising from the level of a vertebral body above. The pleural line may be distinctly thicker in obese patients due to excessive extrapleural fat. This condition can usually be differentiated from pleural disease by its perfect symmetry.
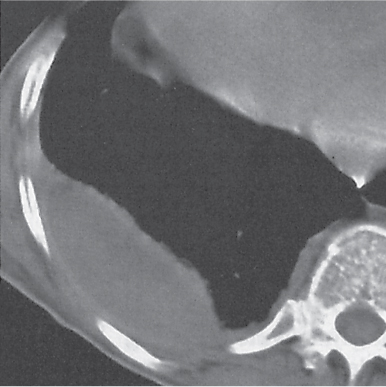
A pneumothorax (Fig. 17.1) is easily diagnosed on CT scans even in the presence of extensive soft tissue emphysema. Other air collections, such as subpleural blebs, pneumopericardium, and mediastinal emphysema, can also readily be differentiated from an adjacent loculated pneumothorax with CT.
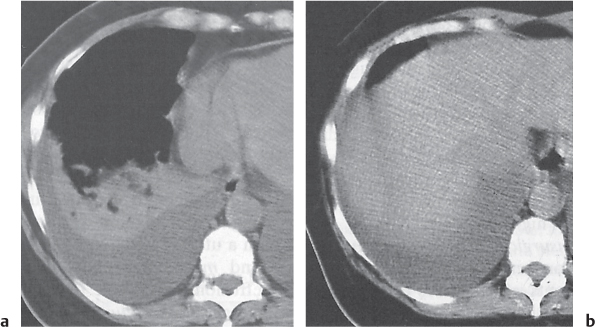
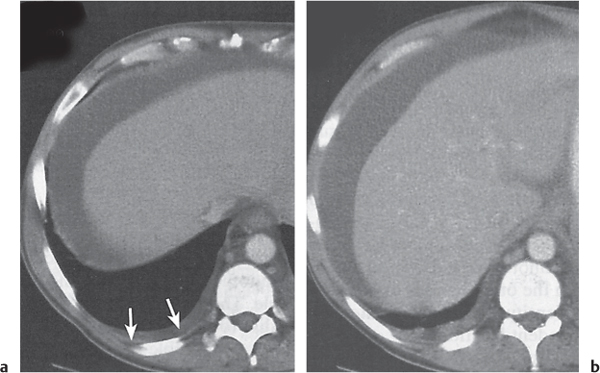
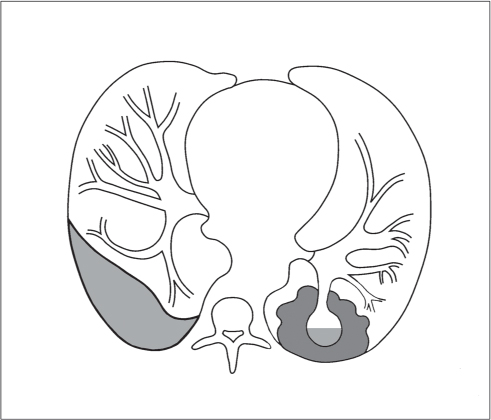
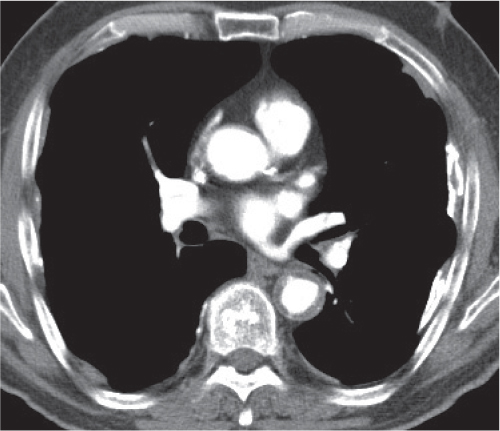
Pleural effusions (transudative, exudative, and chylous) have a homogeneous, near-water density and cannot be differentiated by CT (Fig. 17.2). An acute hemothorax, by contrast, commonly appears rather inhomogeneous, with a CT density greater than water, especially in its most dependent locations (hemoglobin). Pleural fluid initially collects in the most dependent portion of the pleural space, which is posteromedial and caudal to the lung base in the supine position. Small amounts of fluid usually appear crescent- or lenticularlike, but it may also be impossible to differentiate a discrete pleural effusion from pleural thickening. In these cases, freely mobile fluid can be diagnosed by obtaining an additional set of images in a prone or lateral decubitus position. Furthermore, after intravenous contrast medium administration, a thickened, inflamed, or neoplastic pleura enhances, whereas purely fibrotic pleural thickening and pleural fluid do not. Larger pleural effusions extend toward the lateral chest wall and may enter the major fissure, where the fluid tapers medially, producing a characteristic “beak” sign. Large pleural effusions may also compress the lower lobe and displace it anteriorly. The collapsed lower lobe thus seems to float on the pleural fluid. In this situation, the noninflated posterior edge of the lower lobe may easily be mistaken for the diaphragm with pleural fluid posteriorly and peritoneal fluid anteriorly. Similarly, inversion of a hemidiaphragm by massive pleural effusion may also simulate intra-abdominal fluid. However, a correct diagnosis usually is possible by analyzing the relationship of both fluid collection and the lower lobe on subsequent transverse images and multiplanar reformations.
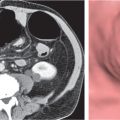
Both pleural and peritoneal fluid presents as an arcuate or semilunar density displacing liver and spleen inward from the adjacent chest wall (Figs. 17.2 , 17.3). The entity of the fluid collections can be assessed based on a variety of different criteria. Fluid outside (i.e., peripheral to) the diaphragm is always pleural, whereas fluid inside the diaphragm is peritoneal. Pleural fluid may surround the lung, whereas peritoneal fluid may be surrounded by the lung bases. In the posterior costophrenic angle, pleural fluid is posterior to the diaphragm, causing anterolateral displacement of the crus, whereas peritoneal fluid is anterior to the diaphragm. When scrolling through transverse images in a craniocaudal direction, pleural fluid gradually diminishes, whereas peritoneal fluid increases in size, progressively extending lateral to the liver and spleen. Fluid seen posterior to the liver is within the pleural space, as the peritoneal space does not extend into this region (the bare area of the liver is not covered by peritoneum). The interface of pleural fluid with the liver or spleen is hazy, whereas with peritoneal fluid, it is sharp.
Unilateral or bilateral pleural effusions not associated with any other signs of intrathoracic disease are often tuberculous in younger patients and predominantly neoplastic in the elderly. Neoplastic effusions are found with metastases, lymphoma, and leukemia and in the Meigs–Salmon syndrome. The latter describes a nonmalignant pleural effusion and ascites in the presence of benign or malignant ovarian tumors, or occasionally a uterine leiomyoma. Pleural effusions may also be the sole finding of viral or mycoplasmatic infections. Regarding collagen vascular diseases, both rheumatoid disease and systemic lupus erythematosus (primary and drug-induced) often present with pleural effusion as the only intrathoracic manifestation. Congestive heart failure is the most common cause of pleural effusion, but it usually is associated with an enlarged cardiac silhouette and other signs of cardiac decompensation. In Dressler syndrome, pericardiac and pleural effusions typically develop 2 to 3 weeks after myocardial infarction or pericardial surgery. However, occasionally they may occur months or even years after the causative episode.
Traumatic and postsurgical pleural effusions are common, but both history and associated findings are usually diagnostic. Patients with asbestos exposure occasionally present with pleural effusion alone. A variety of abdominal diseases, such as pancreatitis, subphrenic abscess, ascites of any etiology, renal failure, and cirrhosis, are frequently associated with pleural effusion, but in these conditions, the primary cause is usually clearly evident on CT. Myxedema, familial Mediterranean fever (familial paroxysmal polyserositis), and primary lymphedema are rare inherited conditions presenting with pleural effusion as the only intrathoracic abnormality.
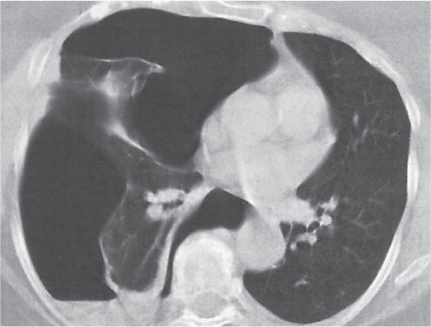
Empyema is a purulent pleural infection usually secondary to a bacterial pneumonia. Other less frequent extrapulmonary sources include bacteremia, subphrenic abscess, spondylitis, thoracotomy, and penetrating chest trauma. An empyema has to be differentiated from a parapneumonic effusion, which is an uninfected (sympathetic) serous exudate in pneumonia that resolves spontaneously. It results from increased permeability of the inflamed visceral pleura. Pulmonary infections that frequently extend beyond the pleural space into the chest wall include actinomycosis and nocardiosis, and occasionally tuberculosis (TB), blastomycosis, and coccidioidomycosis. Large empyemas may compress the neighboring lung, resulting in gradual displacement and bowing of the adjacent pulmonary vessels and bronchi. The visceral and parietal pleural layer appears relatively thin, smooth, and of uniform thickness but strongly enhances on postcontrast scans. Nonenhancing pus thus becomes clearly visible between both pleural layers (“split pleura” sign). The shape of the empyema, as well as a possible air–fluid level, changes when moving the patient from a supine to a prone or decubitus position.
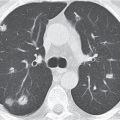
A loculated empyema (Fig. 17.4) is trapped between the visceral and parietal pleura and thus is lenticular, elliptical, or round on cross-sectional images. It is sharply demarcated from lung parenchyma and forms an obtuse angle with the chest wall.
In an organizing empyema, the walls may become thickened and eventually even calcified. Demonstration of fluid collections within the thickened pleural peel is highly suspicious of a still ongoing and active infection.
An empyema has to be differentiated from a peripheral lung abscess abutting the pleural surface (Fig. 17.5). Lung abscesses tend to be spherical without change in shape and with equi-dimensional air–fluid level when the patient is scanned in different positions. The margins of a lung abscess are irregularly shaped, poorly defined, and may contain areas of cavitation. The lesion characteristically forms an acute angle with the chest wall. Pulmonary vessels and bronchi seem to enter the abscess rather than being displaced by it. On postcontrast scans, the walls of a lung abscess usually strongly contrast with the necrotic low-density material in the center.
Pleural thickening is primarily caused by fibrosis and neoplasia. Pleural fibrosis is a common sequela of hemothorax, empyema, TB, and exposure to asbestos or talc. In all these conditions, focal to extensive pleural calcifications are frequent, and, with the exception of asbestos and talc inhalation, the pleural fibrosis predominantly affects the visceral pleura. Other causes of nonneoplastic pleural thickening are fungal infections, chronic polyarthritis, radiation therapy, organizing pleural effusions, sarcoidosis, and, rarely, splenosis (posttraumatic implantation of splenic tissue on the left pleura). Neo-plastic pleural thickening is associated with mesotheliomas (benign and malignant), metastasis, lymphoma, local invasion from bronchogenic carcinomas (especially Pancoast tumors), and malignant thymomas.
Extensive pleural thickening caused by fibrosis may result in encasement of the lung, resulting in restriction and loss of volume. A thickened layer of extrapleural fat often becomes visible at this stage, separating the parietal pleura, which may be calcified, from the rib cage.
Asbestos-related pleural disease (Figs. 17.6 , 17.7) is almost invariably bilateral. Pleural plaques (smooth focal thickening of the parietal pleura) are characteristically visible adjacent to the posterior and lateral inner surface of the sixth to tenth ribs. The pleura between adjacent ribs is often spared (“skip lesions”). Diffuse, more or less uniform pleural thickening in the lower hemithorax is another less frequent manifestation. Focal visceral pleural fibrosis may occur and cause interlobular fissural thickening, occasionally simulating pulmonary nodules or arousing a round atelectasis. Pleural plaques with or without calcifications frequently are evident in the diaphragmatic pleura but typically spare the costophrenic angles. Calcifications range from punctate, nodular, or linear densities to complete encirclement of the lower portions of the lung. Diaphragmatic plaques may occasionally be missed on transverse CT slices if they lie in the scanning plane. Mediastinal and paravertebral pleural plaques and thickening also are common CT findings.
Benign (fibrous) mesotheliomas (Fig. 17.8) usually arise from the visceral pleura. They present as a localized, sharply defined soft tissue mass, sometimes with slightly lobulated margins, ranging from 2 to 14 cm in diameter. Smaller lesions are homogeneous on CT, whereas larger lesions may have an inhomogeneous appearance, particularly on postcontrast scans, due to necrosis, hemorrhage, cyst formation, and calcification. Occasionally, a small pleural effusion is associated.
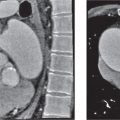
Malignant mesothelioma (Figs. 17.9 , 17.10) is a highly malignant neoplasm and carries an extremely poor prognosis. The majority of cases are found in patients with known asbestos exposure. It presents as diffuse nodular or plaquelike pleural thickening that eventually encases the entire lung. Hemorrhagic pleural effusions are commonly associated and may mask irregular, nodular pleural thickening caused by the malignancy. CT images obtained from different scan positions (e.g., prone and supine or lateral decubitus) usually allow pleural fluid collection to be differentiated from mesothelioma. In the presence of malignant mesothelioma, even large effusions frequently do not cause significant mediastinal shifting to the contralateral side, most likely due to tumor encasement of the affected hemithorax. Significant contrast enhancement of mesotheliomas is often helpful to differentiate tumor from both asbestos-related pleural thickening and loculated pleural effusions. The tumor typically spreads by local invasion into fissures and adjacent structures, such as the ipsi-lateral lung, mediastinum, pericardium, diaphragm, lateral chest wall, and contralateral hemithorax. Hematogenous metastases are uncommon.
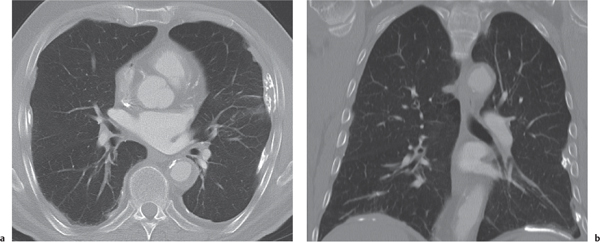
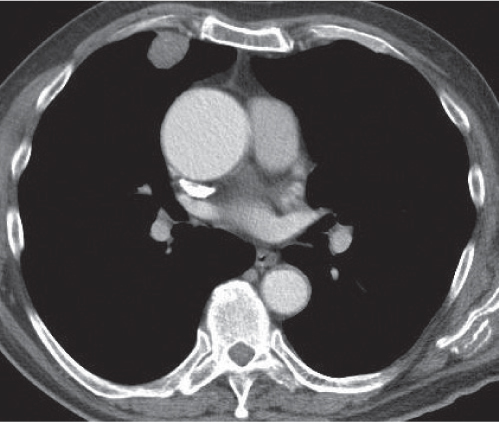
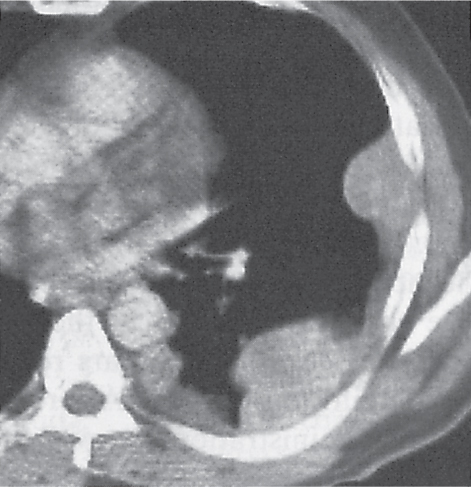
Pleural metastases (Figs. 17.11 , 17.12) presenting with nodular pleural thickening and effusions are often indistinguishable from malignant mesothelioma. Bilateral involvement, as well as the absence of both asbestos-related pleural disease and pulmonary asbestosis, favors metastatic disease. Besides bronchogenic carcinoma (especially Pancoast tumor) and malignant thymoma, which both invade the pleura by contiguous spread, hematogenous pleural metastases most frequently originate from carcinoma of the breast, kidney, ovary, and gastrointestinal tract. Pleural lymphoma may present as a localized, broad-based pleural mass, usually associated with pleural effusion, but is rarely an initial manifestation of the disease.
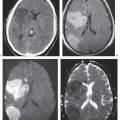
An apical cap is a crescent-shaped soft tissue equivalent mass at the lung apex with or without a superior sulcus lesion that may be caused by a variety of conditions. Pleural fluid tends to accumulate over the pulmonary apex, especially in the supine position. Apical pleural thickening not associated with a soft tissue mass may result from chronic TB (usually bilateral but often asymmetric), healed empyema, hemothorax, or radiation therapy. Any irregular soft tissue mass with involvement of the brachiocephalic plexus and vessels and/or invasion of the adjacent ribs and vertebral bodies is suspicious of a malignant neoplasm. The most common superior sulcus malignancy is a Pancoast tumor (Fig. 17.13), but metastases (e.g., from breast carcinoma; Fig. 17.14) and, rarely, lymphoma can present in a similar fashion. Benign tumors are rare and may be of either neural (e.g., neurofibroma and schwannoma) or mesenchymal (e.g., fibroma, desmoid, and lipoma) origin. Hematomas and hemorrhages in the superior sulcus are either associated with rib, clavicle, and spine fractures, secondary to aortic or great vessel rupture, or iatrogenic (e.g., catheter perforation). Atelectasis of the upper lobe and normal variants, such as excessive periapical fat or vascular anomalies (e.g., elongation or dilation of the subclavian artery), may also produce apical densities, but they are easily differentiated on CT scans due to their characteristic features.
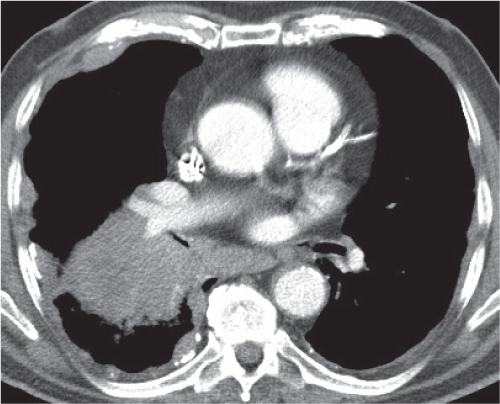
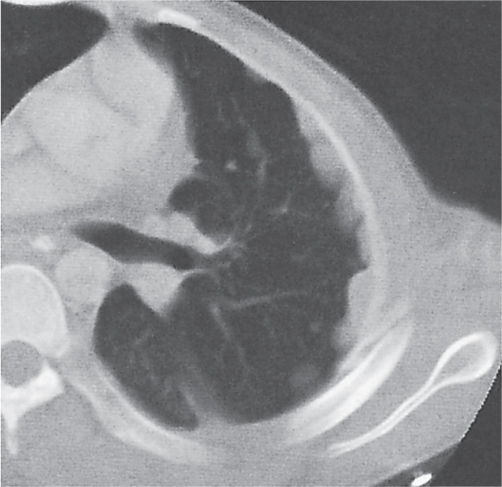
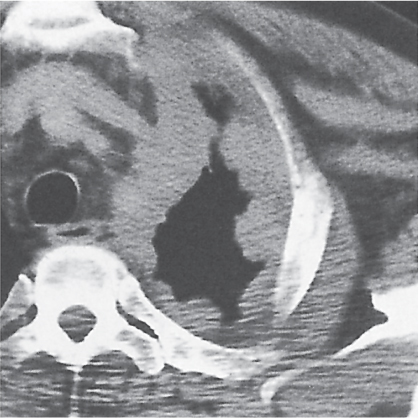
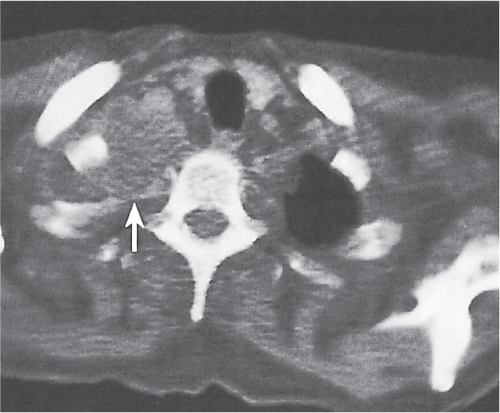
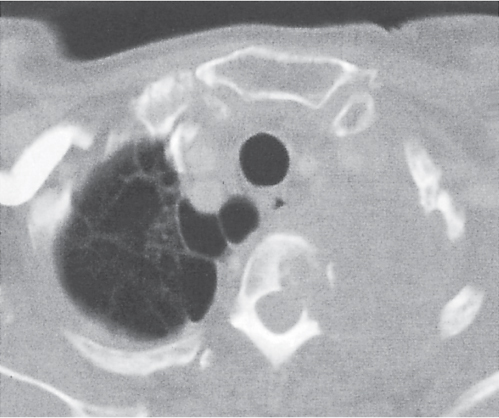
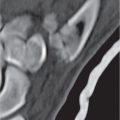
Extrapleural chest wall lesions commonly originate from the ribs or intercostal soft tissues, including vessels and nerves. Displacing the overlying parietal and visceral pleura centrally, these lesions typically form an obtuse angle with the chest wall (Fig. 17.15). Associated chest wall abnormalities (e.g., rib destruction) support their extrapleural origin. Rib lesions are most commonly caused by traumatic, neoplastic, and infectious processes and do not differ from their presentation in other skeletal locations. Common rib lesions include healed fractures, metastases (Fig. 17.16), multiple myeloma (Fig. 17.17), osteomyelitis (bacterial, tuberculous, or fungal), and benign conditions, such as fibrous dysplasia, bone cyst, enchondroma, osteochondroma, eosinophilic granuloma, brown tumors, and extramedullary hematopoiesis (Fig. 17.18).
The axillary space is confined by the pectoralis major and minor muscles anteriorly; the latissimus dorsi, teres major, and subscapularis muscles posteriorly; the chest wall and serratus anterior muscle medially; and the coracobrachialis and biceps muscles laterally. When scanning is performed in a supine position, both arms are lifted above the head, and the axilla is thus bare to the side.
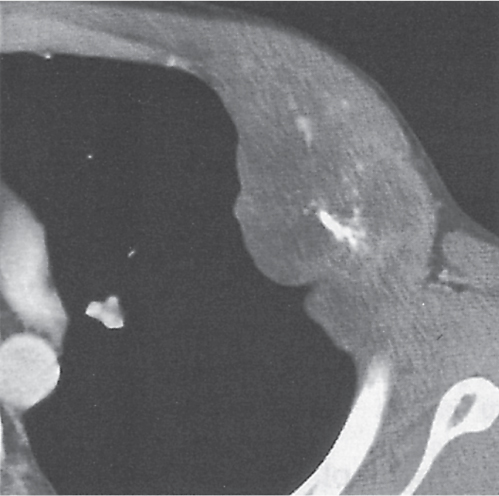
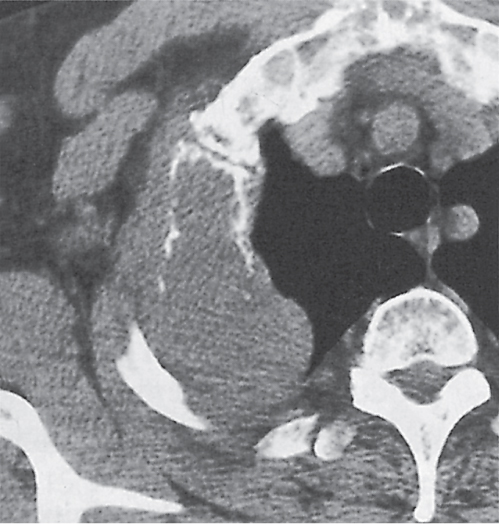
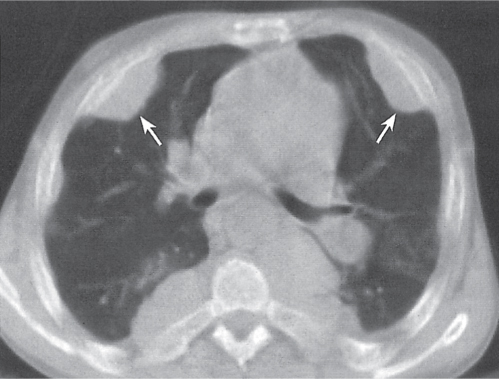
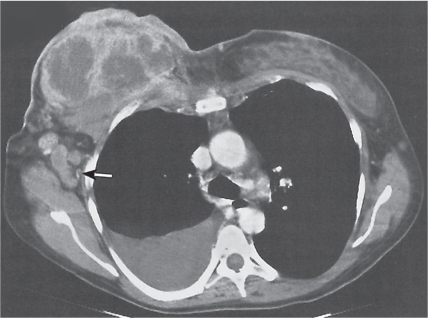
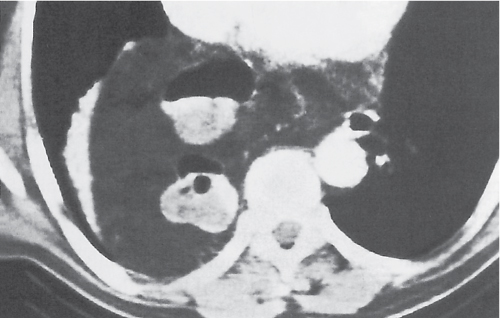
The axilla contains the axillary artery and vein, branches of the brachial plexus, and a large number of lymph nodes all embedded within the fatty tissue. The axillary vein lies physiologically anterior and caudal to the axillary artery, whereas the brachial plexus is located cephalad and posterior to the artery. Normal axillary lymph nodes are oval shaped, measure < 10 mm in size, and contain a central fatty hilus. Ovoid lymph nodes measuring between 1.5 and 2 cm in diameter suggest inflammation (reactive hyperplasia). Lymph nodes exceeding 2 cm in diameter are indicative of metastatic or lymphomatous disease. A common origin of axillary and parasternal (i nternal mammary) lymph node metastases is breast carcinoma (Fig. 17.19). However, lymph nodes may appear rather small but still bear micrometastases. As a general rule, the ratio between smallest and largest lymph node diameter should be ≤ 0.5. Any ratio approaching 1—and thus corresponding to a rounding of the node—is highly suspicious of inflammatory, infectious, or metastatic affection. Lack of central fat is likewise pathognomonic, but it may be observed in asymptomatic persons.
The diaphragm is a large, dome-shaped muscle that incompletely divides the thorax from the abdomen. The diaphragmatic crura are tendinous structures arising from the anterolateral surface of the upper lumbar spine. The right crus is larger and longer than the left crus and originates from the first three lumbar vertebral bodies, whereas the left crus arises from the first two lumbar vertebrae. The nodular appearance of the diaphragmatic crura should not be mistaken for enlarged retrocrural lymph nodes, which normally are small (< 6 mm in diameter).
Diaphragmatic hernias may be either congenital or acquired. Hiatal hernia (herniation of the stomach through the esophageal hiatus) is by far the most common type and does not require CT for diagnosis. Bochdalek hernias (Fig. 17.20) are commonly left-sided and occur anywhere along the posterior costodiaphragmatic margin. They tend to be rather large and may contain omental or retroperitoneal fat, bowel, spleen, liver, kidney, stomach, and pancreas. Morgagni hernias are rare and usually right-sided; tend to be small; occur through an anteromedial parasternal defect; may contain liver, omentum, or bowel; and are often associated with a pericardial defect.
Traumatic diaphragmatic hernias (Fig. 17.21) are found with both blunt and penetrating trauma, but they can be asymptomatic for months and even years. Over 90% of these hernias are located on the left side, usually in the central or posterior portion of the diaphragm. Omentum, stomach, bowel, spleen, and kidney may herniate through the ruptured diaphragm; thus, strangulation is a common complication.
A diaphragmatic eventration is caused by a congenitally weak diaphragm with cephalad displacement of the corresponding abdominal content. The eventration occurs more frequently on the right side, where it involves the anteromedial portion of the diaphragm. In this situation, the liver is displaced superiorly and should not be confused with a peripheral pulmonary or pleural mass. On the left side, the eventration usually involves the entire hemidiaphragm and mimics diaphragmatic paralysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree