18 Cholangiocarcinoma
Aladin T. Mariano, R. Peter Lokken, and Charles E. Ray Jr.
18.1 Introduction
Cholangiocarcinoma is an aggressive neoplasm of the biliary epithelium associated with significant morbidity and mortality. Diagnosis and treatment of cholangiocarcinoma require a multidisciplinary approach that involves diagnostic and interventional radiologists, gastroenterologists, surgeons, pathologists, and medical oncologists. Interventional radiologists play an increasingly essential role in the diagnosis and management of this devastating disease. The following sections will provide an overview of cholangiocarcinoma, including the epidemiology, pathology, diagnosis, and treatment options for this disease.
18.2 Epidemiology
Cholangiocarcinoma is the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC). 1 On histology, cholangiocarcinoma is most commonly seen as adenocarcinoma with associated fibrous stroma formation (> 90%); however, there are rare variants, such as squamous/adenosquamous, clear cell, undifferentiated, mucinous/signet ring, and lymphoproliferative types, among others. 2 , 3 Numerous risk factors for cholangiocarcinoma have been established, including primary sclerosing cholangitis (PSC), choledochal cysts, chronic viral hepatitis, liver flukes, and hepatolithiasis, although most patients with cholangiocarcinoma have no known risk factors. 4
The incidence of cholangiocarcinoma varies significantly by geographic region and is highest where hepatobiliary infection with liver flukes is endemic. Thailand has one of the highest annual incidences of cholangiocarcinoma at 96 cases per 100,000; in the United States, the annual incidence is less than 3 cases per 100,000. 5 The disease typically presents in the seventh decade of life, with men at slightly higher risk than women. 6 The most common presenting symptoms include jaundice (84%), weight loss (35%), and abdominal pain (30%). 6
Cholangiocarcinoma is often categorized anatomically as intrahepatic or extrahepatic. Gallbladder carcinoma, a bile duct epithelial neoplasm, is considered a separate category that has its own management guidelines. 7 Intrahepatic cholangiocarcinoma (ICC) is defined as involving the second-order (or beyond) peripheral bile ducts. Extrahepatic cholangiocarcinoma (ECC) includes perihilar tumors involving the confluence of the left and right hepatic ducts and tumors involving the more inferior common hepatic and common bile ducts. 8 In the United States, the annual incidence of ICC is less than that of ECC, at 0.58 per 100,000 compared to 0.88 per 100,000, although the relative incidence of ICC may be increasing. 9 The Surveillance, Epidemiology, and End Results (SEER) program has reported an increase in the age-adjusted annual incidence of ICC from 0.13 per 100,000 in 1973 to 0.85 per 100,000 in 1995 to 1999 and a concurrent decrease in the incidence of ECC from 1.08 per 100,0000 in 1979 to 0.82 per 100,000 in 1998. 8 Survival is affected by the anatomic distribution at diagnosis. ICC has the best 5-year survival rate of 40%, whereas distal and perihilar ECC have 5-year survival rates of 23 and 10%, respectively. 6
The prognosis and treatment of cholangiocarcinoma can be further stratified by tumor morphology. The Liver Cancer Study Group of Japan classifies tumors as mass-forming, periductal (infiltrating or sclerosing), intraductal (polypoid or papillary), or mixed mass-forming periductal types. 10 Mass-forming types have a worse prognosis; the intraductal type has a relatively favorable prognosis. 11 , 12 Because the papillary type does not infiltrate into the submucosal layer, it is associated with improved survival after resection. 13
Perihilar infiltrating tumors are stratified into four groups by the Bismuth–Corlette system (▶ Fig. 18.1). 14 Type 1 lesions involve the common hepatic duct more than 2 cm inferior to the confluence. Type 2 lesions involve the common hepatic duct less than 2 cm inferior to the confluence. Type 3 and 4 lesions involve the confluence of the hepatic ducts: type 3 lesions involve the confluence and either the right or left hepatic ducts (type 3a lesions involve the right hepatic duct; type 3b lesions involve the left hepatic duct), and type 4 lesions include tumors involving the biliary confluence and both hepatic ducts and multifocal disease.

Mixed hepatocellular carcinoma–cholangiocarcinoma (HCC-CC) was first recognized in 1949 and has been considered rare, although this disease is being recognized with increasing frequency. 15 , 16 In one study of patients who received orthotopic liver transplant (OLT) for HCC, HCC-CC, or ICC was present in 10 of 302 explants (3.3%); compared to matched controls with only HCC on explant, patients with HCC-CC had a poorer prognosis with a high rate of tumor recurrence after OLT. 16 On imaging, patients with HCC-CC may demonstrate HCC-predominant, cholangiocarcinoma-predominant, or mixed enhancement characteristics. 17 The HCC component typically enhances during the arterial phase and hypoenhances during the portal venous or delayed phases. Cholangiocarcinoma-predominant mixed disease typically shows gradual enhancement. On magnetic resonance (MR) imaging, combined tumors typically demonstrate moderate hyperintensity on T2-weighted images, and contrast dynamics on enhanced images are similar to those seen on computed tomography (CT). 18 , 19
18.3 Diagnosis of Cholangiocarcinoma
The diagnosis of cholangiocarcinoma may be suggested by clinical evidence of cholestasis and hepatic failure, the presence of risk factors, and elevations in the tumor markers carbohydrate antigen 19–9 (CA 19–9) and carcinoembryonic antigen (CEA). However, the clinical presentation is generally nonspecific, and both tumor markers can be elevated by other causes such as other malignancies, nonmalignant biliary obstruction, and liver injury. Imaging and biopsy are therefore often required to establish the diagnosis. 7 , 20
18.3.1 Imaging
Ultrasound
Transabdominal ultrasound is frequently performed in patients with cholangiocarcinoma to evaluate biliary obstruction. Periductal or intraductal cholangiocarcinoma may manifest as indirect findings of biliary dilation and parenchymal atrophy if obstruction is long standing. Although the inciting tumor is often not discernible, the site of reduction in biliary caliber may indicate its location. Perihilar infiltrating lesions may appear as a mass disrupting the confluence of the right and left hepatic ducts and possibly involving adjacent vascular structures. 21
Mass-forming ICC often appears as a solid hypovascular mass with irregular, well-defined margins or as abnormal liver echotexture. The mass may be hypoechoic, hyperechoic, or mixed, without specific features to differentiate it from other solid hepatic lesions. 3 , 21 Further evaluation with CT, MR imaging, or positron emission tomography (PET) fused with CT (PET/CT) is generally needed to evaluate the extent of intrahepatic and extrahepatic disease.
Computed Tomography
Contrast-enhanced multiphase CT is superior to ultrasound in characterizing the extent of intrahepatic, extrahepatic, and distal metastatic disease in patients with cholangiocarcinoma. Intraductal tumors may present as ductal ectasia with or without discernible plaquelike or papillary intraluminal tissue that enhances mildly on arterial and portal venous phases. Periductal infiltrating tumors tend to manifest as periductal concentric and irregular thickening and enhancement with associated biliary obstruction. 3
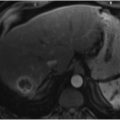
Mass-forming cholangiocarcinoma is typically hypoattenuating on noncontrast phase images with an incomplete, thin rim of enhancement on arterial and portal venous phases and with varying degrees of progressive central enhancement on delayed phase images (▶ Fig. 18.2). 22 Peripheral enhancement correlates to viable neoplasm on pathology, whereas progressive central enhancement represents contrast diffusion into the fibrous interstitial spaces of desmoplastic tissue. 3 , 23 , 24 Ancillary features such as capsular retraction secondary to fibrosis, satellite nodules, bile duct dilation, and vascular encasement support the diagnosis of cholangiocarcinoma. Segmental atrophy may also occur secondary to chronic biliary or portal venous obstruction. 25

Magnetic Resonance Imaging
MR imaging also plays a role in the diagnosis and characterization of cholangiocarcinoma. Mass-forming lesions are typically hypointense or isointense to liver on T1-weighted images and mildly to moderately hyperintense on T2-weighted images peripherally (▶ Fig. 18.3). 26 After administration of gadolinium contrast, mass-forming lesions demonstrate an enhancement pattern similar to that seen on multiphase CT, with early marginal enhancement and progressive enhancement of desmoplastic components. Depending on the degree of necrosis, the central T2 signal can range from low to high in intensity. Central T2 shortening may correlate to severe fibrosis on pathology. 27

MR imaging with cholangiopancreatography (MRCP) is a valuable adjunct for evaluation of the biliary tree. MRCP is superior to CT in evaluating intraductal tumors and has an efficacy similar to that of CT in delineating extension to the vasculature and lymph nodes. 21 , 28 MRCP imaging has an efficacy similar to that of percutaneous transhepatic cholangiography in determining the longitudinal extent of hilar cholangiocarcinoma. 29
Positron Emission Tomography
18F-fluorodoxyglucose PET/CT is helpful in determining management strategies and prognosis for patients with cholangiocarcinoma. 30 Identifying the status of the lymph nodes is critical, as this is an important prognostic factor in patients with R0 resection margins. 6 PET/CT has demonstrated higher accuracy than CT in the diagnosis of regional lymph nodes metastases (75.9 vs. 60.9%; p = 0.004) and distant metastases (88.3 vs. 78.7%; p = 0.004). 31 In one study, approximately 24% of patients had a change in surgical or medical management after undergoing PET/CT. 32
18.3.2 Endoscopic Retrograde Cholangiopancreatography
Gastroenterologists perform endoscopic retrograde cholangiopancreatography (ERCP) to access the biliary tree with retrograde cholangiography, allowing access for biopsy and stent placement. The retrograde cholangiogram may identify malignant features of strictures, such as a long segment or shelf-like margin; however, the specificity of this technique is low, as 5 to 25% of these strictures may be benign on histologic analysis. 33 , 34
18.3.3 Biopsy
Although imaging is effective in noninvasive characterization of the disease extent, biopsy is often required for diagnostic confirmation of cholangiocarcinoma. 1 , 7 Common approaches include laparoscopic biopsy, endoscopic fine-needle aspiration (FNA) and cytologic brushings, and percutaneous biopsy.
ERCP enables biliary tree access and visualization and allows for tissue sampling. Endoscopic brush cytology is often performed, although this technique has a relatively low sensitivity of approximately 58%; 35 other studies have reported an accuracy of 9 to 24%. 21 Endoscopic ultrasound (EUS) may be used to visualize hilar lesions and evaluate the involvement of regional lymph nodes and vessels. 1 FNA biopsy with EUS of the distal bile duct lesions has been shown to have a sensitivity of 77 to 89% and a specificity of 100%. 36 , 37 , 38 Although tumor seeding after FNA is of concern, particularly in cases of potentially resectable disease, this event appears to be exceedingly rare, occurring at a rate of approximately 1 in 10,000 to 40,000 biopsies. 1
Percutaneous image-guided biopsy is particularly valuable in cases of mass-forming lesions and can be performed under ultrasound or cross-sectional imaging guidance depending on lesion conspicuity and operator preference (▶ Fig. 18.4). In one study, malignant lesions measuring 3 cm or smaller were accurately sampled by core-needle biopsy (18-gauge automated side-cutting needles through a 17-gauge introducer package) in 84.4% of cases and by FNA in 91.7% of cases. 39 Smaller lesions measuring 1.5 cm or less were associated with similar success rates. 40 , 41

For intraductal lesions without a targetable mass-forming component, percutaneous brush cytology or forceps biopsy may be used. The sensitivity of transhepatic brush cytologic biopsy in the evaluation of malignant strictures ranges from 30 to 75%. 42 , 43 Forceps biopsy provides a sample of subepithelial tissue and has been reported to have a higher sensitivity compared to brush biopsy (43–81% or higher). 43 , 44
In patients with established transhepatic biliary tracts from prior percutaneous drainage, an endobiliary core biopsy can be performed, although this method is less established. A cohort of 18 patients with suspicious biliary stricture underwent 19-gauge, 2-cm core biopsy through a transhepatic sheath and inner 14-gauge curved metal cannula. 45 Sensitivity and specificity were reported as 76 to 81% and 100%, respectively.
18.4 Management of Cholangiocarcinoma
Management of cholangiocarcinoma often requires a multidisciplinary approach that involves interventional radiologists, radiation oncologists, surgeons, medical oncologists, and gastroenterologists. Interventional radiologists play an important role in providing symptomatic relief with biliary decompression and in controlling the tumor with transcatheter therapies and thermal ablation.
18.4.1 Drainage Procedures
Percutaneous Biliary Drainage
Percutaneous transhepatic biliary drainage (PTBD) to decompress the biliary tree is indicated for cholangitis, hyperbilirubinemia, and hepatic dysfunction related to malignant obstruction that cannot be treated endoscopically with internal biliary stent placement (▶ Fig. 18.5). 46 , 47 Although ERCP with internal stent placement is generally considered first-line therapy, percutaneous drainage, particularly in perihilar cholangiocarcinoma, has been reported to be as efficacious as endoscopic decompression with a lower risk of complications such as cholangitis. 48 , 49 , 50 PTBD may also be considered in cholangiocarcinoma to relieve liver function test derangements precluding systemic chemotherapy or potentially curative resection. 51 , 52

Patients undergoing percutaneous cholangiography (PTC) and PTBD placement are at risk for periprocedural sepsis. Patients with a fever, history of prior biliary instrumentation, and bilioenteric anastomoses are at particularly high risk for positive bile cultures. Therefore, the Society of Interventional Radiology (SIR) recommends the use of preprocedural antibiotics. 53 Intravenous antibiotics that cover organisms such as Enterococcus should be administered before PTBD, and patients should be closely monitored for at least 6 hours after the procedure for signs of sepsis. 54 Antibiotics may be terminated 24 hours after placement in the absence of clinical signs of infection. Overdistention of the biliary tree with contrast during PTC and PTBD may also increase the risk of bacterial translocation and septicemia and should be avoided.
Placement of an internal/external PTBD that traverses the site of obstruction and terminates within the small bowel is generally preferable to the use of external drainage, as an internal/external PTBD allows physiological drainage and the preservation of bile salts. The internal/external drain can be attached to gravity drainage to maximize decompression. Alternatively, the catheter may be capped for internal physiologic biliary drainage, which allows for the preservation of bile salts, prevention of associated electrolyte imbalance, and improved quality of life. If the stricture cannot be traversed, an external PTBD that terminates peripheral to the site of obstruction may be placed and attached to gravity drainage.
The patient should be monitored for signs of catheter dysfunction such as fever, disproportionate abdominal pain, drainage from the skin entry site, and liver function test abnormalities to prompt catheter evaluation and possible exchange. In the absence of clinical symptoms, catheters are generally exchanged every 4 to 6 weeks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree