18 Ultrasound-Guided Interventional Procedures: Biopsy and Drainage
Ultrasound (US) guidance is ideal for most interventional procedures in children because it provides high-resolution, real-time imaging without exposure to ionizing radiation. Computed tomography (CT) and magnetic resonance (MR) imaging are rarely required for pediatric interventions; however, CT (or cone beam CT) may be helpful in some complex or difficult procedures, or procedures in the lungs, posterior or middle mediastinum, or bone.
The radiologist planning and performing the procedure must have appropriate background knowledge; children are not simply small adults, and errors will occur if all of the factors specific to childhood disease and imaging are not considered. Multidisciplinary team discussions should be routine before most tumor biopsy or drainage procedures, especially when the procedure is complex. Such discussions may need to take into account the relative strengths of an institution, with input from surgery, radiology, and pathology.
Several studies have demonstrated that US guidance is safer and more successful than a “blind” approach. This applies to both targeted and nontargeted biopsy procedures. Image guidance during targeted biopsy procedures allows a better evaluation of the lesion and increases the diagnostic yield. Even in nontargeted biopsy procedures, the use of real-time US may be vital in planning a safe approach and identifying local complications immediately. The use of US during drainage procedures usually avoids the need for a fluoroscopic assessment of guidewire or catheter position, thereby eliminating or significantly reducing the radiation dose. High-frequency US probes show the needle tip position in exquisite detail, which may be vital, for example, in small collections or nondilated urinary systems.
Possible complications of US-guided biopsy or drainage include hemorrhage, infection, and pneumothorax; air embolism, bile leak, and perforation of hollow viscera are rare but important risks to consider. Percutaneous interventions can cause unsuspected bleeding into the thorax or abdomen, often distant from the site of access. This can lead to delayed recognition of complications postoperatively. It is vital that the medical and nursing teams caring for the child understand exactly what procedure has been performed and are aware of the importance of regular observations, so that any deterioration in the child’s status can be managed immediately.
The seeding of malignant cells along a percutaneous biopsy needle track, although very rare, is of particular concern because the consequences can be significant. The incidence of tumor seeding is unknown because it can be difficult to distinguish from local recurrence, particularly when a new tumor develops in the parenchyma of the involved organ rather than in adjacent tissues.
18.1 Biopsy
18.1.1 Techniques and Equipment
Most needle biopsy procedures in children are nontargeted biopsies of the liver, kidney, or spleen, or biopsies of tumors or other soft-tissue masses. Occasionally, targeted biopsies of focal nonmalignant lesions of the liver, spleen, or kidney are requested, such as in patients with fungal disease. The basic principles of biopsy, described below, apply in all cases.
The patient should be adequately imaged before the procedure. In cases in which the lesion may be difficult to biopsy, the operator should consider whether there are alternative sites of disease that are simpler to access, such as involved lymph nodes, or fluid that may yield positive cytology or microbiology results on aspiration. Pediatric tumors are often very large at presentation, and much of the lesion may consist of nonviable necrotic tissue or fluid. In such cases, tissue viability must be fully evaluated by contrast-enhanced CT or MR imaging, diffusion-weighted MR imaging, or fluorodeoxyglucose F 18 positron emission tomography (18F-FDG-PET). An on-table assessment of the lesion with US alone may not provide reliable information regarding tissue viability and may lead to a nondiagnostic biopsy. In general, a positive yield is most likely to be obtained from tissue at the margins of a large lesion.
In general, nontargeted biopsies of the liver or kidney can be performed in older children and teenagers under sedation alone, but most other biopsies should be performed under general anesthesia. This allows the operator to work at an unhurried pace, with careful attention to detail and without patient movement or anxiety. The operator must discuss patient positioning and paralysis with the anesthesiologist before the procedure. Prone positioning should be avoided when possible because it increases the risk and complexity of the anesthetic technique. When it is desirable to be able to suspend respiration for a short time during a biopsy procedure, an intravenous bolus of propofol may be effective, but when prolonged or repeated “breath-holds” are required, it may be better to use neuromuscular blockade with endotracheal intubation and positive pressure ventilation.
Informed consent must be obtained by the operator or by a delegate who is familiar with the benefits and risks of the procedure, its technical aspects, and any relevant alternative options. Acceptable preoperative coagulation parameters differ according to the type of biopsy. In general, superficial soft-tissue lesions in children who are otherwise well can be biopsied without a prior evaluation of the coagulation parameters or platelet count. Before biopsies in the abdominal and thoracic cavities, these parameters should be checked and corrected when necessary. Consideration should be given to maintaining normal levels in the postoperative period if delayed bleeding is considered a risk (e.g., liver biopsy following a bone-marrow transplant).
Procedures should be performed in a familiar US machine in a room where the maintenance of sterile conditions can be ensured. Most pediatric biopsies can be performed with a high-frequency linear array transducer; in some cases—for example, in native kidney biopsies in teenagers—a lower-frequency curvilinear array transducer is required.
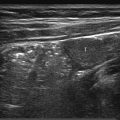
Fine needle aspiration cytology has very few advantages in children, and core needle biopsy is almost always preferred. Various needle types are available, but most operators prefer semiautomated cutting needles ( Fig. 18.1 ). These have a tip that is manually extended under US guidance before the needle is fired, trapping a core of tissue in the cutting notch. Variable throw needles have a cutting notch of adjustable length, and these are very useful for small lesions or those that lie very close to the organ capsule or are adjacent to vital structures. The diameter of the needle selected depends on the nature of the biopsy ( Table 18.1 ).

Organ or tissue | Nature of biopsy | Needle gauge |
Liver | Nontargeted | 18 |
Liver tumor | 18 | |
Kidney | Nontargeted | 16 |
Renal tumor | 16 | |
Spleen | Nontargeted or focal lesion | 18 |
Other abdominal organs | Bowel | 18 |
Suspected neuroblastoma | 14–16 | |
Lung | Focal lesion | 18 |
Soft tissue | Suspected vascular anomaly | 16–18 |
Neuroblastoma or benign tumor | 14–16 | |
Other malignant tumor | 16 | |
Bone | Soft tissue component | 14–16 |
Lymph node | Confirmation of metastasis from known primary tumor | 18 |
Identification of causative organism in infection | 18 | |
Primary diagnosis of lymphoma | 14–16 |
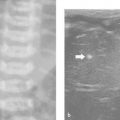
More than one tissue core will be required in most pediatric biopsies. For this reason, coaxial systems are becoming more popular. In these systems, the outer (access) needle is usually 1 gauge larger than the inner (biopsy) needle. There are three main advantages of using a coaxial technique ( Fig. 18.2 ). First, the capsule of the organ or lesion is breached only once, reducing the risk for complications. Second, an unlimited number of cores can be obtained from different parts of the lesion by angling the outer needle. Third, the biopsy track can be blocked at the end of the procedure—for example, by injecting gelatin sponge (as plugs or a slurry) through the outer needle—in order to reduce the risk for bleeding and tumor seeding, although there is little evidence to support that this is effective. To avoid insufficient sampling, it is important to communicate with the pathologist who will examine the biopsy specimen to determine the minimum number of core samples required. A liver biopsy for staging viral hepatitis may require only one core, whereas a biopsy for suspected neuroblastoma may require 10 to 15 cores. Using a coaxial technique in the latter case is clearly mandatory.


Tips from the Pro
Focal renal or splenic lesions may be easier to visualize with the patient’s breath held in inspiration by the anesthesiologist.
Filling the bladder with fluid may afford better views of the pelvis.
A safe access route to certain lesions—for example, in the adrenal region—may be created by injecting saline to displace adjacent organs.
Challenging US-guided biopsies or drainages may be facilitated by using:
an angiography room with cone beam CT capability;
electromagnetic tracking technology.
Combined US- and cone beam CT-guided procedures may be facilitated by using:
needle guidance software;
image fusion techniques when preprocedural imaging provides information not available with cone beam CT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree