19 Portal Vein Embolization
David Li and David C. Madoff
19.1 Introduction
The anticipated liver remaining after extensive hepatic resection, commonly described as the future liver remnant (FLR), is a critical factor associated with the risk of perioperative liver failure and death. 1 , 2 Hence, the ability to modulate the FLR has become a key component of modern oncologic hepatobiliary surgery practice. 3 Preoperative portal vein embolization (PVE) serves as a well-established means to improve the safety of extensive hepatic resection by redirecting flow to the FLR in an effort to hypertrophy and ultimately improve the functional reserve of the nonembolized segments. 4 , 5 , 6 , 7
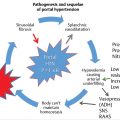
The liver is unique in its ability to regenerate after injury or resection. In the setting of either liver injury or resection, massive hepatocyte proliferation can occur, resulting in recovery of functional liver mass within 2 weeks after the loss of upward of two-thirds of the liver. 8
Regeneration of the liver is dependent on both the stimulus of the injury and the condition of the liver parenchyma. Hepatocyte proliferation is proportional to the severity of the liver injury/resection; minor injuries (< 10% parenchymal involvement) induce only localized mitotic reactions, whereas major injuries (> 50% parenchymal involvement) result in multiple mitotic waves throughout the entire liver. 9 Regeneration rates are dependent on the time from injury, with the greatest rate of regeneration after PVE typically occurring within the first 2 weeks (▶ Fig. 19.1). 10 The predominant mechanism of cell death after PVE is cell-mediated apoptosis rather than the necrosis observed after transarterial embolization (TAE). 11 A direct correlation can be observed clinically: unlike TAE, PVE is typically not associated with postembolization syndrome (characterized by nausea, fever, and pain).

In 1990, Makuuchi et al 12 first reported on the utility of PVE in promoting FLR hypertrophy before hepatic resection in 14 patients with hilar cholangiocarcinoma. Since that time, PVE has continued to gain traction as a well-established technique to modulate the FLR. Alternative techniques have recently been proposed to modulate FLR, including transhepatic liver venous deprivation (LVD) and associating liver partition and portal vein ligation (ALPPS). 3 , 13 , 14 Transhepatic LVD is in its infancy, and only limited reports are available to support its clinical adoption. Proponents of ALPPS suggest that greater, more rapid liver hypertrophy occurs with this technique than with PVE. 14 However, recent studies have raised concerns over increased morbidity and mortality associated with ALPPS as compared to PVE; histologically, the regenerative hepatocytes observed with ALPPS are immature compared to those observed with PVE. 15 These findings suggest that although ALPPS is associated with greater absolute size of FLR, the size does not correlate with greater functional increase. Hence, PVE remains the standard of care at many tertiary care centers, with numerous studies validating its clinical utility to modulate the FLR. This chapter reviews the current indications for and technical aspects of PVE before hepatic resection, with an emphasis on strategies to improve outcomes.
19.2 Clinical Considerations
19.2.1 Indications and Contraindications
PVE allows for safe, potentially curative hepatectomy for patients previously considered ineligible for resection based on anticipated small remnant livers. 6 , 12 , 16 , 17 , 18 , 19 , 20 , 21 Candidates for PVE include hepatic resection candidates with primary or metastatic liver disease who have anticipated FLRs that are too small for adequate function during the perioperative period. If too little liver remains after resection, immediate postresection hepatic failure leads to multisystem organ failure and death. If a marginal volume of liver remains, cirrhotic or not, the lack of reserve often leads to a cascade of complications, prolonged hospital and intensive care unit stays, and slow recovery or slowly progressive liver failure over weeks to months, with eventual death. 1 , 2 , 7
To determine whether a patient will benefit from PVE, several factors must be considered. 5 First, the presence or absence of underlying liver disease will have a major effect on the volume of liver remnant needed for adequate function. A healthy liver has a greater regenerative capacity than a cirrhotic liver, functions more efficiently, and tolerates injury better. Patients can survive resection of up to 90% of the liver in the absence of underlying liver disease, but survival after resection beyond 60% of the functional parenchyma in patients with cirrhosis is unlikely. 16 Second, liver volume is directly correlated with a patient′s size (larger patients require larger liver remnants); hence, standardizing the anticipated liver volume to a patient′s size results in a more accurate assessment of the FLR. 7 , 22 Third, the extent and complexity of the planned resection and the possibility that associated nonhepatic surgery will be performed at the time of liver resection (e.g., hepatectomy plus pancreaticoduodenectomy) must be considered. These three factors are considered in the setting of the patient′s age and comorbidities (e.g., diabetes) that may affect hypertrophy. Thus, once the procedure type and extent of resection necessary to treat the patient have been determined, appropriate liver volumetry is performed so that the standardized FLR (sFLR) volume expressed as a percentage of the estimated total liver volume (TLV) can be used to determine the need for PVE.
Candidates for PVE include patients with primary or metastatic liver disease who would be hepatic resection candidates but for the following factors:
Healthy underlying liver and an sFLR less than 20%. 10 , 16 , 23
Cirrhosis and/or advanced fibrosis and an sFLR less than 40%. 1 , 24
Undergoing extensive chemotherapy and with an sFLR less than 30%. 25
PVE is an adjunctive procedure to major hepatectomy (▶ Fig. 19.2). Hence, contraindications to PVE mirror those of hepatectomy. Severe portal hypertension precluding surgery is the only absolute contraindication to PVE. Also, for cases in which tumor obstructs the portal system in the liver to be resected, PVE is not necessary as portal flow is already redirected to the FLR. 16 , 26 , 27 Relative contraindications include uncorrectable coagulopathy, renal failure, and extrahepatic metastasis. The introduction of two-stage hepatectomy has expanded the population of patients with bilobar hepatic disease burden who are eligible for PVE and potential curative resection; however, diffuse hepatic disease burden remains a contraindication to PVE.

19.2.2 Outcomes
PVE is indicated in patients with healthy underlying liver and sFLR less than 20%. Multiple studies have demonstrated that hepatectomy in a setting of sFLR less than 20% is associated with increased postoperative complications. 10 , 16 , 23 A study of 112 patients by Ribero et al 10 demonstrated that sFLR less than 20% and degree of sFLR hypertrophy after PVE less than 5% predicted a poor outcome after resection (▶ Fig. 19.3). 10 Kishi et al 23 found that in a series of 301 consecutive patients who underwent extended right hepatectomy, those with a preoperative sFLR less than 20% had significantly higher rates of postoperative liver insufficiency and death from liver failure compared with patients with sFLR greater than 20% (p < 0.05). In addition, patients who underwent PVE before surgery to increase their sFLR from less than 20 to greater than 20% had statistically equivalent rates of liver insufficiency as patients with sFLR greater than 20% at baseline (▶ Fig. 19.4). 23 This study confirmed the association between an sFLR threshold less than 20% and increased perioperative complications, as well as the beneficial role of PVE in reducing perioperative complication rates in those patients who undergo liver hypertrophy to an sFLR greater than 20%.


Liver regeneration occurs at a reduced rate and capacity in diseased livers, an observation directly correlated with clinical outcomes. Patients with cirrhosis with marginal liver remnants are not only at high risk for complications but also at increased risk for death from liver failure. 1 As such, the recommended sFLR cutoff in the setting of cirrhosis is 40%, higher than the 20% sFLR cutoff recommended in the setting of healthy underlying liver parenchyma. 6 , 28 , 29 In patients with chronic liver disease, hepatectomy outcomes, including the number and severity of complications and the incidence of postoperative liver failure and death, are better with PVE than without. 24 , 30 , 31 Azoulay et al 30 reported long-term outcomes after resection of ≥ 3 liver segments for hepatocellular carcinoma (HCC) in patients with cirrhosis. PVE was performed when the FLR volume was predicted to be less than 40%, and this procedure led to significant increases in FLR volumes for all embolized patients, correlating with a reduced incidence of liver failure and death without differences in disease-free survival rates. Tanaka et al 31 similarly reported several benefits of PVE in a larger study of patients with HCC and cirrhosis. Disease-free survival rates were similar between patients treated with PVE and those not treated with PVE, but cumulative survival rates were significantly higher in the PVE group than in the non-PVE group. In addition, patients with recurrence after PVE plus resection were more often candidates for further treatments such as TAE, an additional benefit of PVE in the long term. However, the complications of PVE are higher in patients with chronic liver disease than in those with an otherwise healthy liver because of an increased risk of secondary portal vein thrombosis, presumably from slow flow in the portal vein trunk after PVE. 32 , 33
In patients with chronic liver disease such as chronic hepatitis, fibrosis, or cirrhosis, the increase in nonembolized liver volumes after PVE varies (range: 28–46%), and hypertrophy after PVE may take greater than 4 weeks because of slower regeneration rates. 34 , 35 The degree of parenchymal fibrosis is thought to limit regeneration, possibly as a result of reduced portal blood flow. 33 Hence, treatment strategies combining transarterial therapy with PVE are particularly useful in cirrhotic populations to maximize potential liver regeneration and prevent disease progression, as discussed in more detail below.
19.2.3 Role of PVE in Conjunction with Transarterial Therapies
PVE can be combined with other interventional radiology techniques such as TAE (▶ Fig. 19.5) in patients who are not anticipated to have sufficient hypertrophy after PVE alone. 36 , 37 The mechanism of TAE is complementary, as a component of inflammation and necrosis is added to the apoptosis-mediated cell death induced by PVE to stimulate liver hypertrophy. Nagino et al 37 first described the use of TAE to improve FLR volume in two patients with cholangiocarcinoma who demonstrated inadequate hypertrophy after PVE. In both patients, PVE in the setting of underlying liver disease led to negligible hypertrophy of the FLR. After interval TAE, the FLR volume demonstrated an adequate increase, and both patients underwent successful curative resection. With combination therapies, both arterial and portal venous hepatic supplies are embolized, placing patients at greater risk for liver necrosis. In Nagino et al′s original report, only half of the target segments were treated because of the potential risk of hepatic infarction.

TAE is now more commonly performed as a staged procedure before PVE, with an interval of 2 to 3 weeks between the procedures to help prevent hepatic infarction. TAE followed by PVE has been advocated in the setting of cirrhosis complicated by HCC. In this patient population, the rationale for performing TAE before PVE includes preventing tumor progression after PVE, reducing arterioportal shunts that may limit the effectiveness of the subsequent PVE, and boosting the regenerative stimulus in chronically diseased livers. 38 , 39 Using this regimen, Aoki et al 38 demonstrated increased profound tumor necrosis without substantial injury to the noncancerous liver in patients with large HCC and chronically injured livers. Similarly, Ogata et al 39 found an increased incidence of complete tumor necrosis (83 vs. 6%; p < 0.001) and increased 5-year disease-free survival rate (37 vs. 19%; p = 0.041) in patients who underwent trans-catheter arterial chemoembolization and PVE versus PVE alone.
With all combination of TAE and PVE regimens, special care must be taken to reduce the risk of hepatic necrosis; this should include staging the procedures at least 2 to 3 weeks apart. Embolization should not be carried out to complete stasis, and the use of nonparticulate embolic agents such as chemoembolization should be favored over particulate agents. After embolization, the interventional radiologist should confirm patency of the hepatic artery supplying the targeted liver segment to avoid occlusion of arterial and portal hepatopetal flow and the potential of parenchymal necrosis.
19.2.4 Role of Concurrent Chemotherapy
PVE has been reported to accelerate tumor growth for both primary and metastatic liver tumors. 40 , 41 , 42 , 43 Progression of disease after PVE may preclude curative intent surgery; in two-stage hepatectomy series, 20% dropout rates due to progression of disease after first-stage resection have been reported. 44 , 45 Neoadjuvant chemotherapy can be administered to provide tumor control in the interim between PVE and resection; however, concerns have been raised regarding its potential deleterious effects on liver function. Two separate studies have demonstrated an association between chemotherapy agents and sinusoidal dilation and steatohepatitis. 46 , 47 Given these findings, Shindoh et al 25 performed a retrospective analysis of 194 patients with colorectal liver metastasis to determine the optimal FLR in patients treated with neoadjuvant chemotherapy. The authors found that both long duration of chemotherapy (defined as > 12 weeks) and sFLR ≤ 30% were predictors of hepatic insufficiency (odds ratio [OR] = 5.4; p = 0.004; OR = 6.3; p = 0.019, respectively; ▶ Fig. 19.6). 25 No cases of postoperative mortality and only two cases of postoperative hepatic insufficiency were reported when the sFLR is greater than 30%, indicating that an sFLR greater than 30% may be a more appropriate cutoff value in patients who have received neoadjuvant chemotherapy, particularly if the duration of treatment is greater than 12 weeks.

The effect of systemic neoadjuvant chemotherapy on liver hypertrophy after PVE has also been addressed by several studies. 48 , 49 , 50 Zorzi et al 50 reviewed FLR hypertrophy after PVE in patients with colorectal liver metastases who underwent PVE either with concomitant neoadjuvant chemotherapy (n = 43) or without chemotherapy (n = 22) before resection. Patients treated with chemotherapy and those not treated with chemotherapy demonstrated similar rates of hypertrophy at 4 weeks after PVE. Covey et al 48 also reported on patients with colorectal liver metastases who underwent PVE either with (n = 47) or without (n = 53) neoadjuvant chemotherapy and found no significant difference between the groups in the median contralateral liver growth after PVE.
Several studies have examined the effect of chemotherapy on disease progression after PVE performed before hepatectomy. 51 , 52 , 53 , 54 Spelt et al 54 found that the rate of tumor progression in patients who underwent PVE was low when concomitant chemotherapy was administered; this low rate was seen when the interval between completion of chemotherapy and PVE was short. Fischer et al 51 reported on a series of 64 consecutive patients who underwent PVE, including 25 patients who received chemotherapy and 39 who did not, in preparation for extended right hepatic resection. Although there was no statistical difference between the two groups in the proportion of patients who ultimately underwent hepatic resection, the chemotherapy group had a statistically lower rate of progression by Response Evaluation Criteria in Solid Tumors (RECIST) criteria (18.9 vs. 34.2%; p = 0.03). Of greater importance, patients who received chemotherapy demonstrated a clear survival benefit versus those who did not (5-year survival; 49 vs. 24%; p = 0.006) in both the surgical resection and nonsurgical cohorts.
In summary, PVE is likely associated with accelerated tumor growth. Administration of neoadjuvant chemotherapy is helpful in lowering the rate of disease progression and does not interfere with liver regeneration. However, when long-term chemotherapy is administered (> 12 weeks), an sFLR cutoff of greater than 30% should be considered, given the potential deleterious effects of chemotherapy on native liver function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree