Normal variant |
Mild ventricular enlargement can occur without associated cerebral or cerebellar abnormality. |
Ventricular size usually increases with age, and it is most pronounced after 60 years. |
Aqueductal stenosis ( Fig. 2.7 and Fig. 2.8 ) |
MRI: Dilatation of lateral and third ventricles with normal-sized fourth ventricle, ± dilatation of only the upper portion of cerebral aqueduct and not the lower portion, ± discrete or poorly defined lesion in midbrain. Subependymal edema can be seen on FLAIR. |
Aqueductal stenosis can result from a small lesion/neoplasm in the midbrain or debris or adhesions from hemorrhage or inflammatory diseases. It may also result from extrinsic compression of the tectal plate and aqueduct from massively enlarged ventricles or ventricular diverticulum. MRI can exclude other lesions causing obstruction of CSF flow through the aqueduct, such as lesions in the posterior third ventricle or posterior cranial fossa. |
Chiari I malformation ( Fig. 2.9 ) |
Cerebellar tonsils extend more than 5 mm below the foramen magnum in adults, 6 mm in children < 10 years old. Syringohydromyelia occurs in 20 to 40%. Hydrocephalus in 25%. Basilar impression in 25%. Less common associations are Klippel-Feil syndrome and atlanto-occipital assimilation. |
Cerebellar tonsillar ectopia. Most common anomaly of CNS. Not associated with myelomeningocele. |
Chiari II malformation (Arnold-Chiari) ( Fig. 2.10, Fig. 1.12) |
Small posterior cranial fossa with gaping foramen magnum through which there is an inferiorly positioned vermis associated with a cervicomedullary kink. Beaked dorsal margin of the tectal plate. Myelomeningoceles in nearly all patients. Hydrocephalus and syringomyelia common. Dilated lateral ventricles posteriorly (colpocephaly). |
Complex anomaly involving the cerebrum, cerebellum, brainstem, spinal cord, ventricles, skull, and dura. Failure of fetal neural folds to develop properly results in altered development affecting multiple sites of the CNS. |
Chiari III malformation |
Features of Chiari II plus lower occipital or high cervical encephalocele. |
Rare anomaly associated with high mortality. |
Dandy-Walker malformation ( Fig. 2.11 ) |
Vermian aplasia or severe hypoplasia, communication of fourth ventricle with retrocerebellar cyst, enlarged posterior fossa, and high position of tentorium and transverse venous sinuses. Hydrocephalus common. Associated with other anomalies, such as dysgenesis of the corpus callosum, gray matter heterotopia, schizencephaly, holoprosencephaly, cephaloceles, and others. |
Abnormal formation of roof of fourth ventricle with absent or near-incomplete formation of cerebellar vermis. |
Vermian hypoplasia (Dandy-Walker variant) (Fig. 1.43) |
Mild vermian hypoplasia with communication of posteroinferior portion of the fourth ventricle with cisterna magna. No associated enlargement of the posterior cranial fossa. |
Occasionally associated with hydrocephalus, dysgenesis of corpus callosum, gray matter heterotopia, or other anomalies. |
Colpocephaly ( Fig. 2.10 ) |
Asymmetric enlargement of the occipital horns of the lateral ventricles. |
Associated with Chiari II malformation and dysgenesis of corpus callosum. |
Neoplasms (causing obstructive hydrocephalus) |
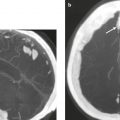
Metastatic tumor ( Fig. 2.12 ) |
MRI: Circumscribed spheroid lesions in brain, can have various intra-axial locations (often at gray–white matter junctions), usually low-intermediate signal on T1-weighted imaging, intermediate-high signal on T2-weighted imaging, ± hemorrhage, calcifications, and cysts. Variable gadolinium contrast enhancement, often with high signal on T2-weighted imaging peripheral to a nodular enhancing lesion representing axonal edema. Metastatic lesions can also involve the skull, dura, leptomeninges, ventricles, choroid plexus, or pituitary gland. Leptomeningeal tumor often best seen on postcontrast images.
CT: Lesions usually have low-intermediate attenuation, ± hemorrhage, calcifications, or cysts. Variable contrast enhancement, often associated with adjacent low attenuation. |
Represents ~ 33% of intracranial tumors, usually from extracranial primary neoplasm in adults more than 40 years old. Primary tumor source: lung > breast > GI > GU > melanoma. |
Intra-axial Primary Tumors |
Pilocytic astrocytoma ( Fig. 2.13 ) |
MRI: Solid/cystic focal lesion with low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging and FLAIR, usually showing gadolinium contrast enhancement. Lesions commonly are located in cerebellum, hypothalamus, optic chiasm, adjacent to third or fourth ventricles, or in brainstem. Thirty percent can have more aggressive MRI features, such as inhomogeneous gadolinium contrast enhancement, central necrotic zones, and irregular margins.
Diffusion-weighted imaging: Usually no evidence of restricted diffusion. Diffusion tensor imaging can show tumor displacement of cortical spinal tracts.
Magnetic resonance spectroscopy: With these low-grade tumors in children, a paradoxical pattern associated with more aggressive tumors (elevated choline/N-acetylaspartate and lactate levels) may be seen.
CT: Solid/cystic focal lesion with low-intermediate attenuation, usually with prominent contrast enhancement. |
Most common glioma in children and accounts for 6% of all gliomas. Slow-growing cystic-solid WHO grade I astrocytoma with biphasic pattern of compacted bipolar cells, Rosenthal fibers, multipolar cells, microcysts, and eosinophilic granular bodies. Associated with BRAF mutations involving the MAPK signaling pathway. Usually lack IDH mutations. Immunoreactive to GFAP and apolipoprotein D. Can occur in cerebrum, cerebellum, brainstem, and optic chiasm. In children, 67% occur in the cerebellum and usually have a favorable prognosis if totally resected. Increased occurrence in patients with neurofibromatosis type 1. Leptomeningeal tumor dissemination is rare (< 3%). |
Diffuse astrocytoma |
MRI: Intra-axial lesion with slightly ill-defined margins or diffuse mass lesion in white matter with low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging, ± mild gadolinium contrast enhancement. relative cerebral blood volume (rCBV) is usually low. There is minimal associated mass effect, and it usually lacks peritumoral edema and hemorrhage.
Diffusion-weighted imaging: Tumors typically do not show restricted diffusion.
CT: Focal or diffuse mass lesion usually located in white matter with low-intermediate attenuation, ± mild contrast enhancement. Minimal associated mass effect. |
Low-grade astrocytoma (WHO grade II) that accounts for 10 to 15% of astrocytic brain tumors. Often occurs in children and adults (20–40 years old). Can also occur in children and older adults. Tumors comprised of well-differentiated fibrillary or gemistocytic neoplastic astrocytes. Association with TP53, isocitrate dehydogenase-IDH1 and IDH2, and telomere maintenance protein-ATRX mutation. Mean survival is 6 to 8 years; may undergo malignants progression to anaplastic astrocytoma or glioblastoma. Tumors with IDH mutations have a better prognosis than those without. |
Anaplastic astrocytoma ( Fig. 2.14 ) |
MRI: Often irregularly marginated lesion located in white matter with low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging, ± gadolinium contrast enhancement. Tumors can have elevated relative cerebral blood volume rCBV).
Diffusion-weighted imaging: Tumors typically do not show restricted diffusion.
Magnetic resonance spectroscopy shows decreased N-acetylaspartate (NAA) and high levels of choline.
CT: Irregularly marginated mass lesion with mixed low and intermediate attenuation, ± hemorrhage, prominent heterogeneous contrast enhancement, and peripheral edema. Can cross corpus callosum. |
Malignant astrocytic tumor (WHO grade III) between diffuse astrocytoma and glioblastoma multiforme. Malignant astrocytes have nuclear atypia and mitotic activity. Ki-67/MIB-1 ranges from 5 to 10%. Can progress to glioblastoma. Approximate 2-year survival. Promotor methylation inactivates the effects of the DNA repair enzyme MGMT enabling improved tumor response to alkylating chemotherapy agents. Patients with IDH mutations as well as those who have MGMT promotor methylation have improved responses to chemotherapy. |
Glioblastoma multiforme |
MRI: Irregularly and poorly marginated mass lesion with necrosis or cyst, mixed signal on T1-weighted imaging, heterogeneous high signal on T2-weighted imaging, ± hemorrhage, and prominent heterogeneous gadolinium contrast enhancement. Increased relative cerebral blood volume (rCBV) is associated with high-grade gliomas from tumor-induced angiogenesis. Other findings include peripheral edema and tumor extension across the corpus callosum.
Diffusion-weighted imaging: Tumors typically do not show restricted diffusion.
Magnetic resonance spectroscopy shows decreased N-acetylaspartate (NAA) and high levels of choline.
CT: Irregularly marginated mass lesion with necrosis or cyst, mixed low and intermediate attenuation, ± hemorrhage, prominent heterogeneous contrast enhancement, and peripheral edema. Can cross corpus callosum. |
Glioblastoma multiforme is the most common primary CNS tumor and accounts for 15% of intracranial tumors and up to 75% of astrocytic neoplasms, with an incidence of 3 per 100,000. Most patients are over 50 years old. These highly malignant (WHO grade IV) astrocytic neoplasms have nuclear atypia with increased mitotic activity, cellular pleomorphism, necrosis, and microvascular proliferation and invasion. Ki-67/MIB-1 ranges from 15 to 20%. Associated with mutations involving RTK/phosphatase–PTEN/PI3K signal pathway, TERT, and TP53 and Rb1 tumor suppressor genes. Promotor methylation resulting in inactivation of the MGMT DNA repair enzyme enables improved malignant tumor response to chemotherapy in patients lacking IDH mutations. The extent of lesion is underestimated by MRI; survival is often often < 1 year. Patients with secondary type glioblastoma who have IDH mutations can have a better response to chemotherapy and prognosis than those without. |
Intra-axial Primary Tumors |
Subependymal giant cell astrocytoma (tuberous sclerosis) (Fig. 1.49) |
MRI: Circumscribed lesion located near the foramen of Monro with mixed low-intermediate signal on T1-weighted imaging and mixed high signal on T2-weighted imaging, ± cysts and/or calcifications, + heterogeneous gadolinium contrast enhancement.
Diffusion-weighted imaging: Subependymal giant cell astrocytomas usually do not have restricted diffusion. Magnetic resonance spectroscopy: Subependymal giant cell astrocytomas can have elevated choline and reduced N-acetylaspartate (NAA).
CT: Circumscribed lesion with mixed low-intermediate attenuation, ± cysts and/or calcifications, + heterogeneous contrast enhancement. |
Subependymal giant cell astrocytoma near foramen of Monro, occurs in 6 to 18% of patients with tuberous sclerosis, patients usually younger than 20 years (mean age = 11 years), slow-growing lesions composed of neoplastic astrocytes of varying sizes with nuclear pleomorphism and low Ki-67/MIB-1 ranging from 1.5 to 7.4%. Immunoreactive to GFAP, S-100 protein, neurofilament proteins, and class III b-tubulin. Enlarging lesions can progressively cause obstruction of CSF flow through the foramen of Monro. Autosomal dominant mutations involving TSC1 gene on chromosome 9q34 and TSC2 gene on chromosome 16p13.3. Prevalence of tuberous sclerosis = 1/6,000 newborns. Long-term survival is usual if the tumor is resected. |
Medulloblastoma (primitive neuroectodermal tumor of the cerebellum) ( Fig. 2.15 ) |
MRI: Circumscribed or invasive lesions involving the cerebellum, with low-intermediate signal on T1-weighted imaging, intermediate-high signal on T2-weighted imaging, ± cystic or necrotic zones. Variable gadolinium contrast enhancement and usually high relative cerebral blood volume (rCBV), ± gadolinium contrast enhancement in the leptomeninges and/or ventricles from tumor dissemination. Can cause obstructive hydrocephalus.
Diffusion-weighted imaging: Solid portions can have restricted diffusion.
Magnetic resonance spectroscopy usually shows elevated choline, lipid, and taurine peaks, and decreased N-acetylaspartate (NAA) levels.
CT: Circumscribed or invasive lesions with intermediate to slightly high attenuation, variable contrast enhancement, and frequent dissemination into the leptomeninges |
Highly malignant tumors (WHO grade IV) located in the cerebellum and that frequently disseminate along CSF pathways. Tumors are composed of poorly differentiated or undifferentiated cells with divergent differentiation along neuronal, astrocytic, or ependymal lines. Histologic subtypes include classic, desmoplastic, large cell (LC), anaplastic, and medulloblastoma with extensive nodularity. LC and anaplastic types have the worst prognoses. Typically occurs in patients from 4 weeks to 20 years old (mean age = 5.5 years). Can also occur in adults. |
Atypical teratoid/rhabdoid tumors ( Fig. 2.16 ) |
MRI: Circumscribed or invasive mass lesions with intermediate signal on T1-weighted imaging, ± zones of high signal from hemorrhage on T1-weighted imaging, and variable mixed low, intermediate, and/or high signal on T2-weighted imaging. Solid portions can have restricted diffusion, usually with prominent gadolinium contrast enhancement ± heterogeneous pattern. Gadolinium contrast enhancement may occur in the leptomeninges and/or ventricles from tumor dissemination.
Diffusion-weighted imaging: Solid portions can have restricted diffusion.
Magnetic resonance spectroscopy usually shows elevated choline, lipid, and lactate peaks, and decreased N-acetylaspartate (NAA) levels.
CT: Circumscribed mass lesions with intermediate or mixed low and intermediate attenuation, ± zones of hemorrhage, cystic degeneration, and/or necrosis. Calcifications in tumors occasionally occur. Usually there is contrast enhancement, ± heterogeneous pattern, ± contrast enhancement in the leptomeninges and/or ventricles from tumor dissemination. |
Rare malignant tumors (WHO grade IV) involving the CNS usually occurring in the first decade, commonly before 3 years of age. Ki-67/MIB-1 is often high, > 50%. Associated with mutations of the INI1(hSNF5/SMARCB1) gene on chromosome 22q11.2. Histologically appear as solid tumors ± necrotic areas, similar to malignant rhabdoid tumors of the kidney. Nearly all are intra-axial and can occur in supra- and/or infratentorial locations. Associated with a very poor prognosis. |
Ependymoma ( Fig. 2.17 ) |
MRI: Circumscribed, lobulated, supratentorial lesion, often extraventricular, ± cysts, calcifications, and/or hemorrhage. Low-intermediate signal on T1-weighted imaging, intermediate-high signal on T2-weighted imaging, variable gadolinium contrast enhancement. Tumors have elevated relative cerebral blood volume (rCBV) as well as delayed contrast retention secondary to intratumoral fenestrated blood vessels. Diffusion-weighted imaging usually shows no restricted diffusion.
Magnetic resonance spectroscopy: Elevated choline and decreased N-acetylaspartate (NAA), similar to other neoplasms.
CT: Circumscribed, lobulated, supratentorial lesion, often extraventricular, ± cysts and/or calcifications (up to 50%), with low-intermediate attenuation and variable contrast enhancement. |
Slow-growing tumor (WHO grade II) comprised of neoplastic cells with monomorphic round/oval nuclei containing speckled chromatin, perivascular pseudorosettes, and ependymal rosettes. Zones of myxoid degeneration, hyalinization of blood vessels, hemorrhage, and/or calcifications may occur within tumors. Ependymomas account for 6–12% of intracranial tumors, with an incidence of 0.22 to 0.29 per 100,000. Occur more commonly in children than in adults; one-third of tumors are supratentorial and two-thirds are infratentorial. Infratentorial ependymomas occur in children ranging in age from 2 months to 16 years (mean age = 6.4 years). Supratentorial ependymomas occur in children and adults. Immunoreactive to glial fibrillary acidic protein (GFAP), S-100, vimentin, and/or epithelial membrane antigen (EMA). Associated with neurofibromatosis type 2 and genetic mutations involving chromosomes 22, 9, 6, and 3. Usually lack mutation of IDH gene. Survival is 57% at 5 years and 45% at 10 years. |
Anaplastic ependymoma ( Fig. 2.18 ) |
MRI: Irregularly marginated mass lesion with necrosis or cyst, mixed signal on T1-weighted imaging, heterogeneous high signal on T2-weighted imaging, ± hemorrhage, calcifications, heterogeneous gadolinium contrast enhancement, and peripheral edema. Can disseminate within the CSF.
CT: Irregularly marginated mass lesion with necrosis or cyst, mixed attenuation, ± hemorrhage, calcifications, heterogeneous contrast enhancement, and peripheral edema. Can disseminate within the CSF. |
Malignant glioma (WHO grade III) with ependymal differentiation and high mitotic activity, microvascular proliferation, and pseudopalisading necrosis. Associated with poor prognosis in patients with prominent anaplastic features, CSF metastases, and incomplete resection. |
Hemangioblastoma |
Circumscribed tumors usually located in the cerebellum and/or brainstem.
MRI: Small gadolinium-enhancing nodule ±cyst, or larger lesion with prominent heterogeneous enhancement ± flow voids within lesion or at the periphery; as well as intermediate signal on T1-weighted imaging, intermediate-high signal on T2-weighted imaging. Occasionally, lesions have evidence of recent or remote hemorrhage. Relative cerebral blood volume (rCBV) of lesions is high.
CT: Small contrast-enhancing nodule ±cyst, or larger lesion with prominent heterogeneous enhancement, ± hemorrhage. |
Slow-growing, vascular tumors (WHO grade I) that involve the cerebellum, brainstem, and/or spinal cord. Account for 1–3% of intracranial neoplasms and usually occur in middle-aged adults. Rarely occur in children except for those with von Hippel-Lindau (VHL) disease. Tumors consist of numerous thin-walled vessels as well as large, lipid-containing vacuolated stromal cells that have variably sized, hyperchromatic nuclei. Mitotic figures are rare. Stromal cells are immunoreactive to VEGF, vimentin, CXCR4, aquaporin 1, carbonic anhydrase, S-100, CD56, neuron-specific enolase, and D2–40. Vessels typically react to a reticulin stain. Reactive astrocytic gliosis can occur in adjacent tissue. Tumors occur as sporadic mutations of the VHL gene or as an autosomal dominant germline mutation of the VHL gene on chromosome 3p25–26, resulting in VHL disease. In VHL disease, multiple hemangioblastomas involving the CNS occur as well as clear-cell renal carcinoma, pheochromocytoma, endolymphatic sac tumor, neuroendocrine tumor, adenoma of the pancreas, and epididymal cystadenoma. VHL disease occurs in adolescents and young and middle-aged adults. |
Intraventricular Tumors |
Choroid plexus papilloma ( Fig. 2.19 ) |
MRI: Circumscribed and/or lobulated lesions with papillary projections, intermediate signal on T1-weighted imaging and mixed intermediate-high signal on T2-weighted imaging, usually with prominent gadolinium contrast enhancement, ±calcifications. Locations: atrium of lateral ventricle (children) > fourth ventricle (adults), rarely other locations, such as third ventricle. Associated with hydrocephalus from CSF overproduction or mechanical obstruction.
CT: Circumscribed and/or lobulated lesions with papillary projections, intermediate attenuation, usually prominent contrast enhancement, ± calcifications. |
Rare benign (WHO grade I) intraventricular neoplasms derived from choroid plexus epithelium in which a single layer of cuboidal or columnar epithelial cells with round/oval nuclei overlies fibrovascular connective tissue in frondlike patterns. Occurs in lateral ventricle (50%), fourth ventricle/CP angle (40%), third ventricle (5%), and multiple ventricles (5%). Occurs in children and adults. Tumors in the lateral ventricles account for up to 80% of cases in patients less than 20 years old. Lesions in the fourth ventricle are most common in adults. Tumors have very low mitotic activity. Immunoreactive to cytokeratins, vimentin, podoplanin, S-100, and transthyretin. Can be cured by surgical resection. |
Atypical choroid plexus papilloma ( Fig. 2.20 ) |
MRI: Circumscribed and/or lobulated lesions with papillary projections, intermediate signal on T1-weighted imaging and mixed intermediate-high signal on T2-weighted imaging, usually with prominent gadolinium contrast enhancement, ±calcifications. Locations: atrium of lateral ventricle (children) > fourth ventricle (adults), rarely other locations, such as third ventricle, associated with hydrocephalus.
CT: Circumscribed and/or lobulated lesions with papillary projections, intermediate attenuation, usually prominent contrast enhancement, ± calcifications. |
Rare (WHO grade II) intraventricular neoplasms derived from choroid plexus that have some histologic features that overlap those of choroid plexus papillomas, although atypical papillomas can have higher mitotic activity (> 2/HPF), increased cellularity, nuclear pleomorphism, partial loss of the papillary morphology, and/or necrosis. Immunoreactive to cytokeratins, S-100. Occur in children and adults. Can be cured by surgical resection. |
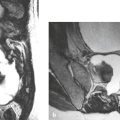
Choroid plexus carcinoma ( Fig. 2.21 ) |
MRI: Large lobulated neoplasms with mean tumor size of 5 cm, often with irregular margins, ±brain invasion. On T1-weighted imaging, tumors have heterogeneous intermediate signal with foci of high signal in 45% from areas of hemorrhage. On T2-weighted imaging, solid portions of the tumor often have heterogeneous intermediate to slightly high signal. Small zones with low signal on T2-weighted imaging can occur from sites of hemorrhage or calcifications. Intratumoral cystic or necrotic zones occur in 64% and have high signal on T2-weighted imaging. Tubular flow voids representing blood vessels occur in up to 55% of tumors. Tumors typically show prominent gadolinium contrast enhancement. Nearly 75% of tumors have irregular enhancing margins from ependymal invasion. Disseminated gadolinium-enhancing tumor in the leptomeninges can occur in up to 45%. Hydrocephalus is seen in up to 80%.
CT: Large intraventricular tumors with intermediate attenuation as well as foci with high attenuation from areas of hemorrhage or calcification, low attenuation zones from cystic or necrotic zones. Tumors typically show prominent contrast enhancement. |
Rare malignant (WHO grade III) intraventricular neoplasms derived from choroid plexus epithelium that contain irregular sheets of neoplastic cells with nuclear pleomorphism, high mitotic activity (> 5/HPF), loss of the papillary morphology, and necrotic and/or hemorrhagic areas. Immunoreactive to cytokeratins. Account for 0.1% of all intracranial tumors and 0.6% of primary pediatric CNS neoplasms. These tumors are five times less frequent than choroid plexus papillomas. Median ages range from 12 to 32 months. Most commonly occur in the lateral ventricle, followed by the fourth and third ventricles. These tumors commonly disseminate along CSF pathways and invade brain tissue. Poor prognosis, with 5-year survival of 45%. |
Subependymoma ( Fig. 2.22 ) |
MRI: Tumors typically attached to ventricular walls (fourth ventricle, 40–50%; lateral ventricle, 30–40%; third ventricle, 10%). Lesions have circumscribed margins, low-intermediate signal on T1-weighted imaging, and heterogeneous slightly high to high signal on T2-weighted imaging. May contain sites of hemorrhage or calcification. Variable degrees of gadolinium contrast enhancement.
CT: Lesions have circumscribed margins, low-intermediate attenuation. May contain sites of hemorrhage or calcification. Variable degrees of contrast enhancement. |
Slow-growing low-grade (WHO grade I) glial neoplasms comprised of clusters of tumor cells with isomorphic nuclei within a dense matrix of cell processes. Mitotic activity is absent or rare. Immunoreactive to glial fibrillary acidic protein (GFAP). Account for 8% of ependymal tumors. Complete resection can be curative. |
Meningioma ( Fig. 2.23 ) |
Extra-axial dura-based lesions that are well circumscribed. Locations are supra- >infratentorial, parasagittal > convexity > sphenoid ridge > parasellar > posterior fossa >optic nerve sheath > intraventricular.
MRI: Dura-based tumors with intermediate signal on T1-weighted imaging, intermediate to slightly high signal on T2-weighted imaging, usually prominent gadolinium contrast enhancement, often with a dural tail, ± calcifications. Intratumoral hemorrhage and cystic or necrotic foci can occur in 15%.
Diffusion-weighted imaging/diffusion tensor imaging: ADC values vary among the different subtypes of meningioma. Some tumors can show restricted diffusion, although these findings can be seen with both benign and atypical tumors.
Magnetic resonance spectroscopy can show elevated alanine (1.5 ppm), lactate, choline, and glutamine/glutamate levels, and reduced N-acetylaspartate (NAA).
CT: Tumors have intermediate attenuation, with or without calcifications, with or without hyperostosis, and usually show prominent contrast enhancement. |
Most common extra-axial tumor, accounts for up to 26% of primary intracranial tumors. Annual incidence is 6 per 100,000, typically occurs in adults (> 40 years old), women > men. Composed of neoplastic meningothelial (arachnoidal or arachnoid cap) cells. Meningiomas are usually solitary and sporadic, but can also occur as multiple lesions in patients with neurofibromatosis type 2. Eighty percent of meningiomas are benign (WHO grade I), although 15% have atypical features (WHO grade II) and ~ 5% have anaplastic histologic features (WHO grade III). Can occur secondary to radiation treatment, with latencies ranging from 19 to 35 years. Classified into different subtypes, such as: meningothelial, fibrous (fibroblastic), transitional (mixed), psammomatous, angiomatous, atypical, and anaplastic. Meningothelial, fibrous, and transitional meningiomas are the most common intracranial types. Usually show immunoreactivity to epithelial membrane antigen (EMA) and vimentin. Secretory meningiomas are typically immunoreactive to CEA. Associated cytogenetic findings of deletion of chromosome 22. Mutations in the NF2 tumor suppressor gene on chromosome 22 have been found in 60% of sporadic meningiomas. |
Hemangiopericytoma |
MRI: Solitary dura-based tumors ranging from 2 to 7 cm in diameter that have low-intermediate signal on T1-weighted imaging, intermediate to slightly high signal on T2-weighted imaging, usually prominent gadolinium contrast enhancement often with a dural tail, ± calcifications. Intratumoral hemorrhage and cystic or necrotic foci can occur in 30%.
Magnetic resonance spectroscopy: Relative ratios of myo-inositol, glucose, and glutathione with respect to glutamate are higher in hemangiopericytomas than in meningiomas.
CT: Tumors have intermediate attenuation with or without calcifications and usually show prominent contrast enhancement. |
Rare (WHO grade II) neoplasms that account for 0.4% of primary intracranial tumors; 50 times less frequent than meningiomas, Tumors composed of closely packed cells with scant cytoplasm and round, ovoid, or elongated nuclei with moderately dense chromatin. Numerous slitlike vascular channels are seen and are lined by flattened endothelial cells, ± zones of necrosis. Immunoreactive to vimentin (85%), factor XIIIa (80–100%), and variably to Leu-7 and CD34. Associated with abnormalities involving chromosome 12. Typically occur in young adults (mean age = 43 years), males > females. Sometimes referred to as angioblastic meningioma or meningeal hemangiopericytoma, they arise from vascular cells—pericytes. Frequency of recurrence and metastases > meningiomas. |
Intraventricular Tumors |
Central neurocytoma ( Fig. 2.24 ) |
MRI: Circumscribed lesion typically located at margin of lateral ventricle or septum pellucidum with intraventricular protrusion, heterogeneous low and intermediate signal on T1-weighted imaging, heterogeneous intermediate-high signal on T2-weighted imaging, ± calcifications and/or small cysts, and heterogeneous gadolinium contrast enhancement.
Diffusion-weighted imaging: Lesions can show reduced ADC values.
Magnetic resonance spectroscopy: Elevated glycine (3.55 ppm), choline and alanine levels, and decreased N-acetylaspartate (NAA) in these lesions. Rare extraventricular neurocytomas have been reported in the frontal and parietal lobes and sellar region.
CT: Circumscribed lesion located at margin of lateral ventricle or septum pellucidum with intraventricular protrusion, heterogeneous low and intermediate attenuation, ± calcifications and/or small cysts, heterogeneous contrast enhancement. |
Slow-growing rare neuroepithelial tumor composed of uniform round cells with neuronal differentiation. Immunoreactive to synaptophysin and NeuN. Represent ~ 0.5% of intracranial tumors. Patients range from 8 days to 67 years old (mean age = 29 years). Imaging appearance similar to intraventricular oligodendrogliomas. Typically benign tumors (WHO grade I) with favorable prognosis after surgery. |
Pineal lesions and tumors ( Fig. 2.25 ) |
MRI and CT findings for the lesions and tumors in the pineal region are listed in detail in Table 1.11. |
Lesions involving the pineal gland include primary pineal tumors (pineocytoma, pineal parenchymal tumor intermediate differentiation, papillary tumor, and pineoblastoma), germ cell tumors, embryonal tumors, and ependymoma, as well as lesions in the pineal region (ependymoma, meningioma, arachnoid cyst, epidermoid, dermoid and lipoma). |
Intraventricular Lesions |
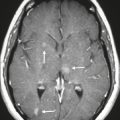
Colloid cyst ( Fig. 2.26 ) |
MRI: Well-circumscribed spheroid lesion located at the anterior upper portion of the third ventricle, with variable signal (low, intermediate, or high) on T1- and T2-weighted imaging, often with high signal on T1-weighted imaging and low signal on T2-weighted imaging. No gadolinium contrast enhancement.
CT: Spheroid lesions located at the anterior upper portion of the third ventricle, with variable attenuation (low, intermediate, or high) and no contrast enhancement. |
Slow-growing, benign cystic lesions whose wall contains a single layer of epithelial cells. Cyst contents can include cholesterol granules, various blood products, macrophages, and various minerals and/or ions. Most colloid cysts occur in the anterosuperior portion of the third ventricle, and rarely in the sella or suprasellar cistern. Usually found in adults (50–60 years old). Can cause acute onset of hydrocephalus. |
Ependymal cyst ( Fig. 2.27 ) |
MRI: Well-circumscribed thin-walled cyst in the lateral ventricles with low signal on T1-weighted imaging, diffusion-weighted imaging, and FLAIR and high signal on T2-weighted imaging. Usually no gadolinium contrast enhancement.
CT: Thin-walled cyst in the lateral ventricles with low attenuation similar to CSF. |
Benign cysts in the lateral ventricles containing serous fluid surrounded by a thin wall containing ependymal columnar cells with vesicular nuclei and eosinophilic cytoplasm. May result from neuroectoderm sequestered during development. Can cause dilation of ventricles. |
Inflammation/Infection |
Ependymitis/ventriculitis ( Fig. 2.28 ) |
MRI: Curvilinear and/or nodular zones of gadolinium contrast enhancement along ventricular/ependymal margins, with resultant communicating or noncommunicating hydrocephalus. Poorly defined zones with high signal on T2-weighted imaging and FLAIR are often seen in the subependymal and periventricular white matter. |
Complications of intracranial inflammatory processes, such as bacterial, fungal, or viral infections, tuberculosis, and parasites. Can result from brain abscess extending into the ventricles and CSF. Noninfectious diseases like sarcoid can result in a similar pattern. |
TORCH and neonatal infections ( Fig. 2.29 and Fig. 2.30 ) |
CT and MRI: Prenatal and neonatal infections often result in intra-axial calcifications. Prenatal infection with cytomegalovirus and rubella can result in microcephaly, schizencephaly, gray matter heterotopia, transmantle clefts, porencephalic cysts, and/or periventricular leukomalacia. Herpes simplex virus and rubella often cause extensive encephaloclastic changes, which result in multicystic encephalomalacia. |
TORCH is an acronym applied to congenital infections from toxoplasmosis, rubella, cytomegalovirus, or herpes. Postnatal viral, bacterial, and fungal infections can result in extensive destructive changes in brain tissue, with compensatory dilatation of the ventricles. |
Cysticercosis ( Fig. 2.31 ) |
MRI: Single or multiple cystic lesions in brain or meninges. Active vesicular phase: Cystic-appearing lesions containing a small 2–4 mm nodule (scolex) with low signal on T1-weighted imaging, FLAIR, and diffusion-weighted imaging; a thin peripheral rim with high signal on FLAIR and T2-weighted imaging; minimal peripheral rim or no gadolinium contrast enhancement; and no peripheral edema on T2-weighted imaging and FLAIR. Active colloidal vesicular phase: Cystic-appearing lesion with low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging, rim and/or nodular pattern of gadolinium contrast enhancement, ± peripheral signal (edema) on T2-weighted imaging. Active granular nodular phase: Cyst retracts into a more solid gadolinium-enhancing granulomatous nodule. Chronic non-active phase: Calcified nodular granulomas. |
Caused by ingestion of encysted larva of the tapeworm Taenia solium in contaminated food (undercooked pork). Involves meninges, subarachnoid space and cisterns > brain parenchyma > ventricles. Most common parasitic disease of the CNS, usually in patients from 15 to 40 years old. Most common cause of acquired epilepsy in endemic regions. Complications include intracranial hypertension from CSF obstruction, arachnoiditis, meningitis, and vascular occlusion. |
Hydatid cyst ( Fig. 2.32 ) Echinococcus granulosus Echinococcus multilocularis |
Echinococcus granulosus
MRI: Single or rarely multiple cystic lesions with low signal on T1-weighted imaging and high signal on T2-weighted imaging surrounded by a thin wall with low signal on T2-weighted imaging, and typically no gadolinium contrast enhancement or peripheral edema unless superinfected. Often located in vascular territory of the middle cerebral artery. Rupture of the hydatid cyst may be contained when only the endocyst ruptures and the pericyst remains intact. In these cases, thickened high signal on T2-weighted imaging is seen in the pericyst from edema and inflammatory reaction, which can show gadolinium contrast enhancement. The ruptured endocyst can have a floating membrane sign or “scroll appearance” with low signal on T1- and T2-weighted imaging within the fluid deep to the pericyst. Rupture of the hydatid cyst beyond the pericyst can also result in an inflammatory host response with complications based on the sites involved.
Echinococcus multilocularis
MRI: Cystic (± multilocular) and/or solid lesions with central zone of intermediate-high signal on T2-weighted imaging surrounded by a slightly thickened rim of low signal, + gadolinium contrast enhancement. Peripheral zone of high signal on T2-weighted imaging (edema) and calcifications are common. |
Rare intracranial lesions caused by parasites Echinococcus granulosus (South America, Middle East, Australia, and New Zealand) or Echinococcus multilocularis (North America, Europe, Turkey, and China). CNS involvement occurs in 1 to 4% of hydatid infections. Humans are intermediate hosts from ingestion of tapeworm eggs in fecally contaminated food or by contact with infected animal tissue. Lesions are often large before becoming symptomatic from raised intracranial pressure. The hydatid cyst has three layers. The outermost layer is the pericyst, which is a thin compressed layer of adjacent host tissue composed of fibrous and inflammatory host cells and usually lacks gadolinium contrast enhancement. The middle layer is the acellular laminated membrane, and the inner layer is the germinative epithelium. The acellular laminated membrane and germinative layer represent the true wall of the cyst, which is referred to as the endocyst. Superinfected hydatid cysts often contain purulent material, commonly with Staphylococcus aureus, and are typically surrounded by an inflammatory reaction in the adjacent brain tissue and/or meninges. |
Rasmussen′s encephalitis ( Fig. 2.33 ) |
MRI: Progressive atrophy of one cerebral hemisphere involving the white matter, basal ganglia, and cortex, with prominent sulci and dilated ipsilateral lateral ventricle. Abnormal high signal on T2-weighted imaging and FLAIR can be seen in the gray and white matter. Usually there is no gadolinium contrast enhancement. |
Uncommon chronic T-cell–mediated immune disorder usually seen in children < 10 years old who have severe and progressive epilepsy and unilateral neurologic deficits, including hemiplegia and psychomotor deterioration. Biopsies show T-cell–dominated encephalitis with activated microglial cells and absence of macrophages, B cells, and plasma cells, and viral inclusion bodies. Treatment is hemispherectomy. |
Neurodegeneration |
Hydranencephaly ( Fig. 2.34 ) |
Replacement of substantial portions of cerebral tissue with thin-walled sacs containing CSF. Inferomedial portions of frontal and temporal lobes are often preserved, and cerebellum and thalami usually have a normal appearance. |
In utero destruction of cerebral parenchyma from injury (vascular, infectious—CMV, toxoplasmosis, etc.). Patients may be normo-, micro-, or macrocephalic. Children are developmentally delayed. |
Porencephalic cyst ( Fig. 2.35 ) |
MRI: Irregular, relatively well-circumscribed zone with high signal on T2-weighted imaging and FLAIR and low signal on T1-weighted imaging and diffusion-weighted imaging similar to CSF, surrounded by poorly defined thin zone of increased signal on FLAIR in adjacent brain tissue. No gadolinium contrast enhancement or peripheral edema.
CT: Irregular, relatively well-circumscribed zone with low attenuation similar to CSF, surrounded by poorly defined thin zone of decreased attenuation in adjacent brain tissue. No contrast enhancement or peripheral edema. |
Represent remote sites of brain injury (trauma, infarction, infection, hemorrhage) occurring in late second trimester with evolution by an encephaloclastic process into a cystic zone with CSF MR signal characteristics surrounded by zones of gliosis in adjacent brain parenchyma. Gliosis (high T2 signal) allows differentiation from schizencephaly. |
Encephalomalacia ( Fig. 2.36 ) |
Poorly defined zone of high signal on T2-weighted imaging in brain tissue (gray and/or white matter) with localized volume loss and compensatory dilatation of adjacent ventricle. |
Damaged residual brain tissue characterized by astrocytic proliferation related to prior infarct, hemorrhage, inflammation, infection, and/or trauma, with compensatory ipsilateral ventricular dilatation resulting from localized volume loss. Encephalomalacia can occur during late gestation, in the postnatal period, or in mature brain when an astrocytic proliferation response is possible. |
Dyke-Davidoff-Mason syndrome ( Fig. 2.37 ) |
Atrophy/encephalomalacia of one cerebral hemisphere, with compensatory dilatation of ipsilateral lateral ventricle, midline shift, unilateral ipsilateral decrease in size of cranial fossa associated with thickened calvarium, ± enlargement of ipsilateral pneumatized paranasal sinuses and mastoid air cells. |
Rare disorder in adolescents presenting with seizures, mental retardation, hemiparesis, or hemiplegia. Can result from congenital injury, such as cerebral infarction from occlusion of the middle cerebral artery, or from postnatal trauma, hemorrhage, ischemia, or infection. |
Alzheimer′s disease ( Fig. 2.38 ) |
MRI: Brain atrophy often most pronounced in the hippocampi and entorhinal cortex of the temporal lobes, thinned gyri, and sulcal and ventricular prominence. Cortical atrophic changes are common. Atrophic changes typically involve the posterior portion of the corpus callosum.
Diffusion tensor imaging: Decreased fractional anisotropy in splenium of the corpus callosum and superior longitudinal fasciculus.
Magnetic resonance spectroscopy: Decreased N-acetylaspartate (NAA)/myo-inositol.
PET/CT: Reduced F-18 FDG activity/glucose metabolism in the parietal and superior/posterior temporal lobes, posterior cingulate cortex, and precuneus.
Increased amyloid-binding radionuclide activity can be seen with PET/CT. |
Alzheimer′s disease is the most common form of progressive dementia and accounts for up to 60% of cases in patients older than 65 years. Pathologic changes include: neurofibrillary tangles from abnormal phosphorylation of microtubule-associated tau protein (tauopathy), senile plaques in gray matter consisting of neurotoxic deposits of amyloid–β 42 protein (deposits also occur in blood vessels), and decreased neurons from neuronal death. Damage begins in the entorhinal cortex and then involves the hippocampus before involving the rest of the cortex. Increased familial risk in 10% of patients related to PSEN1, PSEN2, and APOE*E4 genes. Clinical findings include: poor recent memory; disorientation to time and place; impaired recall, recognition, and working memory; word retrieval difficulty; impaired conversational speech; and impaired object and space perception. |
Frontotemporal lobar degeneration ( Fig. 2.39 ) |
MRI: Progressive brain atrophy often most pronounced in frontal and temporal lobes, hippocampi, and both amygdala; sulcal and ventricular prominence. Cortical atrophic changes common. Abnormal increased signal on T2-weighted imaging can be seen in the subcortical white matter of the atrophied gyri. Atrophy of the anterior portion of the corpus callosum can be seen. |
Frontotemporal lobar degeneration (FTLD) is a heterogeneous group of interrelated neurodegenerative disorders that can be associated with the clinical diagnosis of frontotemporal dementia (FTD). FTD is less common than Alzheimer′s disease. Prevalence of 15 per 100,000 and incidence of 2.2 to 8.9 per 100,000. Pathologic changes include neuronal loss and gliosis as well as deposition of three proteins in the cytoplasm of brain neurons: 1) microtubule-associated protein tau (FTLD-tau), 2) transactive response DNA-binding protein 43 (TDP-43, FTLDTDP), or 3) fused in sarcoma protein (FUS, FTLD-FUS). |
Huntington disease ( Fig. 2.40 ) |
Disproportionate atrophy of basal ganglia (caudate > putamen > cerebellum/brainstem).
MRI: Atrophy of caudate and putamen bilaterally. Variable low signal (iron deposition) or high signal (gliosis) changes on T2-weighted imaging in the putamen bilaterally. Usually no abnormal contrast enhancement.
CT: Progressive atrophy of caudate and putamen bilaterally. |
Autosomal dominant neurodegenerative polyglutamine disease in adults related to abnormal segment (CAG repeats) of DNA on chromosome 4 involving the huntingtin gene. Results in increased synthesis of huntingtin protein, which causes neuronal damage and brain atrophy. Neuronal loss, astrogliosis, and increased oligodentrocytes within a loose textured neuropil are seen in the striatum. Usually presents after age 40 years with progressive movement disorders (choreoathetosis, rigidity, hypokinesia), behavioral abnormalities, and progressive mental dysfunction (dementia). Juvenile Huntington disease also occurs in a small number of patients in their second decade. Patients present with rigidity, hypokinesia, seizures, and progressive mental dysfunction. |
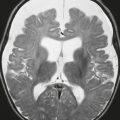
Communicating hydrocephalus, normal-pressure hydrocephalus ( Fig. 2.41 ) |
MRI: Disproportionately greater prominence of the ventricles relative to the sulci, narrowed sulci at the high convexities; ± focal bulging of the roofs of the lateral ventricles; + hyperdynamic CSF flow at the third ventricle, cerebral aqueduct, and fourth ventricle as seen on 2D cine phase contrast MRI and as a flow void on T1- and T2-weighted imaging. Transependymal egress of CSF is also seen as subependymal zones with high signal in T2-weighted imaging and FLAIR.
CT: Disproportionate greater prominence of the ventricles relative to the sulci, with periventricular/subependymal decreased attenuation. Nuclear medicine: In-111 DTPA cisternography can show prominent activity after 24 hours. |
Usually occurs in patients older than 40 years. Dilatation of the ventricles with transependymal egress of CSF is thought to be related to impaired resorption of CSF through arachnoid granulations. Can be associated with progressive memory impairment, urinary incontinence, and gait disorders (normal-pressure hydrocephalus). Patients with normal pressure hydrocephalus have CSF opening pressures between 60 and 240 mm H2O or between 4 and 17 mmHg. Can be idiopathic (primary type) or result from meningitis or subarachnoid hemorrhage (secondary type). Treatment is with ventricular shunting. |
Ventricular shunt failure ( Fig. 2.42 ) |
Enlargement of ventricles above site of CSF outflow obstruction despite the presence of a ventricular shunt catheter. |
Blockage of ventricular shunt catheters can result in progressive ventricular dilation. |