20 Ablation of Malignant Liver Tumors
Ronald S. Arellano
20.1 Introduction
Over the past 15 years, image-guided tumor ablation has evolved to become a well-established therapeutic option for the treatment of liver tumors. 1 , 2 , 3 , 4 , 5 , 6 Thermal ablation offers several distinct advantages over surgical resection including decreased morbidity and mortality, the ability to perform ablations as an outpatient procedure with lower costs, and the potential to offer treatment to patients who might otherwise be poor surgical candidates. Currently available ablation techniques include radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, and irreversible electroporation (IRE; ▶ Table 20.1). Although each modality has its own unique properties, they all share the common goal of generating necrotic tissue through the use of minimally invasive techniques. 7 , 8 , 9 This chapter will describe the currently available ablation techniques and their role in the treatment of liver tumors.
20.2 Ablation Types
20.2.1 Radiofrequency Ablation
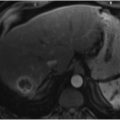
RFA is probably the most studied of the thermal ablation techniques. RFA induces tissue coagulative necrosis via delivery of electromagnetic energy to the patient. This is achieved by placing the patient within a closed-loop electrical circuit that incorporates an RF generator, an electrode applicator, and dispersive electrodes in the form of grounding pads that are placed on the patient. Within this closed-loop circuit, the RF generator creates a high-frequency alternating electrical field within the patient, and high electrical resistance within the tissues leads to ionic agitation as the ions within tissues attempt to align in the direction of the alternating electrical current. This ionic agitation results in friction, which in turn generates heat around the RF electrodes. In most instances, the heat that is generated exceeds 100 °C, which leads to coagulative necrosis (▶ Fig. 20.1). 10 , 11 RF electrode designs range from straight, nondeployable internally cooled electrodes to expandable electrodes. Similarly, treatment algorithms vary among manufacturers, but all have the common feature of achieving tissue necrosis through the application of electromagnetic energy.

20.2.2 Microwave Ablation
MWA (▶ Fig. 20.2; ▶ Fig. 20.3) also uses electromagnetic energy to achieve tissue destruction. With MWA, needles that are inserted into the patient act as antennas that receive electromagnetic energy in the range of 900 to 2,450 MHz. 12 In contrast to RF-induced agitation to induce heat, microwave energy in tissues results in rotation of water molecules within tissues. The rotational movement of water molecules generates frictional heat that then leads to coagulative necrosis. 13 Because an electrical circuit is not created within the patient as with RFA, MWA does not require the use of grounding pads. Currently available microwave devices do not employ internal cooling of antenna or perfusion options.


20.2.3 Cryoablation
Although not commonly used for liver tumor ablation, cryoablation is another thermal ablation option. In contrast to RFA and MWA, cryoablation achieves tissue necrosis by subjecting tissues to temperatures as low as −160 °C. This is accomplished by the circulation of high-pressure argon gas through the lumen of a cryoprobe. Through a thermodynamic process referred to as the Joule–Thomson effect, expansion of the argon gas within the cryoprobe results in tissue cooling. 14 When tissues are subjected to alternating freeze–thaw–freeze cycles, tissue destruction is achieved through different mechanisms. During freeze cycles, ice crystals form within the tissue; this increases osmotic pressure within tumor cells, which leads to cellular dehydration and disruption of cellular membranes, including those of intracellular organelles. 15 , 16 When tissue is subjected to the thaw cycle, the coalescence of ice crystals leads to cellular swelling and further disruption of cellular membranes. The ultimate result is tissue necrosis through cellular apoptosis. One of the limitations of cryoablation is a potentially devastating endotoxin-mediated post-treatment inflammatory response referred to as cryoshock, which is observed in approximately 1% of patients undergoing liver cryoablation and manifests as multi-organ failure and severe coagulopathy. 17 , 18 The cryoshock phenomenon may be associated with mortality rates as high as 18%. 18
20.2.4 Irreversible Electroporation
IRE (▶ Fig. 20.4; ▶ Fig. 20.5), one of the newest ablation devices available for liver ablation, is generally considered to be a non-thermal form of ablation that achieves tissue necrosis through the repetitive delivery of short-duration, high-voltage electrical pulses to tissues. 19 The exposure of tissues to high electrical fields leads to irreversible disruption of cellular membranes, causing tissue necrosis through apoptosis. Because of the strong electrical pulses required, general anesthesia is necessary to achieve complete neuromuscular blockage. Additionally, synchronization between the delivery of electrical energy and the cardiac cycle is necessary to minimize the risk of cardiac dysrhythmias. A potential benefit of the nonthermal nature of IRE is that it is believed to preserve blood vessels and bile ducts, thus enabling treatment near vital structures. 19 , 20 , 21


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree