Infection |
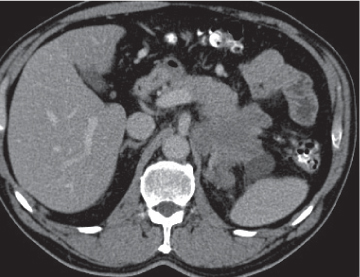
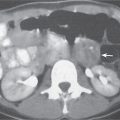
Pancreatic abscess
Fig. 22.6 |
Well-defined cystic lesion in the pancreas.
Scan recommendation:
Biphasic CT (nonenhanced CT, parenchymal phase [PP]).
Nonenhanced CT: Near-water fluid collection. Intracystic gas present in 30% to 50% of cases.
PP: Slight rim enhancement.
Diagnostic pearls: Well-defined cystic mass with intracystic gas in a patient with a known history of pancreatitis. |
Rare, but severe complication of acute pancreatitis. In 30% of cases, there are multiple abscesses. Fistulous tracts are often responsible for intralesional gas collection. Without the presence of gas, abscesses often are indistinguishable from noninfected (pseudo-) cysts. Treatment: PAD, surgery (high mortality without). |
Congenital |
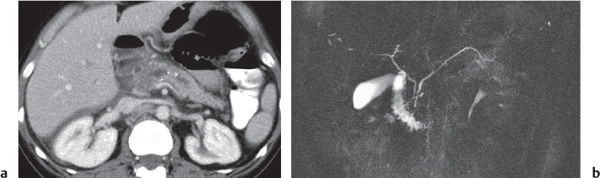
Pancreas divisum
Fig. 22.7a, b |
Nonfusion of dorsal and ventral pancreatic buds.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Large pancreatic head. Separation of ventral and dorsal pancreatic segment sometimes with a visible fatty cleft between both. Nonfusion of ducts visible on thin-slice minimum intensity projection (MinIP).
PP: Hypoattenuation of necrotic areas (if present).
Diagnostic pearls: Large pancreatic head with separate duodenal drainage of nonfused long (dorsal) and short (ventral) pancreatic duct in a patient with recurrent pancreatitis. |
Failure of ventral pancreas in rotation during embryogenesis posteriorly to the duodenum to come (physiologically) into contact with the dorsal pancreas.
Typical clinical symptoms are epigastric pain with or without vomiting in young patients due to recurrent episodes of idiopathic pancreatitis.
Treatment (only in symptomatic patients): sphincteroplasty or surgery.
Usually not diagnosed on CT scans. Magnetic resonance cholangiopancreatography (MRCP) modality of choice. |
Annular pancreas
Fig. 22.8a, b |
Encasement of descending duodenum through ringlike pancreatic tissue.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
NECT: Obstructing band of pancreas tissue around descending duodenum with dilatation of stomach and proximal duodenum.
PP: Better delineation of pancreatic tissue.
Hypoattenuation of necrotic areas (if present). |
Either due to hypertrophy of the dorsal and ventral pancreatic duct or to abnormal migration of the left ventral pancreatic bud to the right of the duodenum rather than to the left.
Typically diagnosed during childhood.
Clinical symptoms are epigastric pain with or without vomiting due to recurrent episodes of idiopathic pancreatitis, but pancreatitis is observed only in 20% to 30% of cases.
Differential diagnosis: pancreatic or duodenal carcinoma. |
Agenesis of dorsal pancreas |
Congenital absence of tail, body, and neck of pancreas.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Normal pancreatic head, absence of tail, and partial to complete absence of body.
PP: No additional information.
Diagnostic pearls: In cases with partial absence of the body, consider pancreatic atrophy. |
Rare. Familial occurrence is indicative of potential hereditary mechanism. Agenesis is most likely a defect of the dorsal pancreatic bud.
Typically asymptomatic, incidental finding. Symptomatic patients may present with recurrent abdominal pain and diabetes mellitus. |
Trauma |
Pancreas laceration
Fig. 22.9a, b |
Traumatic laceration of the pancreas usually affecting either the neck or body.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Hypodense cleft at side of laceration. Blood may appear hyperdense.
PP: No additional information.
Diagnostic pearls: (Hyperdense) peripancreatic fluid in a patient with seat-belt injury. |
Typical complication in car accidents due to seat-belt injuries. Short-lasting but severe encasement between the spine dorsally and the compressed peritoneal cavity ventrally (through the seat belt) in combination with abrupt anteflexion of the body (due to abrupt braking) typically effects laceration within the body of the pancreas.
Laceration is a severe posttraumatic injury that may easily be missed or misinterpreted on initial scans. |
Inflammation |
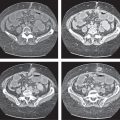
Acute pancreatitis
Fig. 22.10a–c |
Acute inflammation of the pancreas with various etiologies.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Focal diffuse pancreas swelling (edema). Hypodense peripancreatic fluid/hyperdense stranding of peripancreatic fat.
PP: Pancreatic tissue usually enhances homogeneously. Hypodense regions are indicative of necrosis. Rim attenuation is typical for abscess/phlegmon.
Diagnostic pearls: Diffuse swelling of the pancreas, nonenhancing (necrotic) intrapancreatic regions, and peripancreatic fluid collection.
May be accompanied by pleural effusions and fluid along the fascia of Gerota/within the pelvis (space of Douglas). Renal halo sign refers to inflammatory infiltration of the pararenal space with sparing of the perirenal space.
Pancreatic hemorrhage can be seen as hyperdense peripancreatic fluid collection (> 60 HU). |
Etiology includes alcohol abuse, trauma, cholelithiasis, penetrating peptic ulcer, hyperlipoproteinemia, hypercalcemia, and infection.
Clinical severity and CT changes may not correlate. On CT scans, differentiation is possible between edematous (swelling of the pancreas and stranding of peripancreatic fat), exudative (peripancreating fluid collections), and necrotic (hypodense intrapancreatic regions within normal attenuating pancreatic tissue) pancreatitis.
Phlegmonous extension is seen in ~20% of cases (mainly affecting patients with severe symptoms from the start). Any phlegmon may develop into a pseudocyst through progressive liquefaction of contents and development of a fibrous capsule. Hemorrhage/necrosis occurs in ~5% of cases, is associated with high mortality, and usually results from vessel erosion or occlusion.
Pancreatic phlegmon usually extends into the lesser sac or to the left anterior pararenal space, less frequently to the transverse mesocolon and small bowel mesentery. |
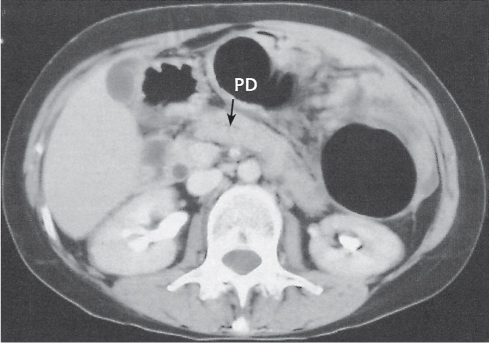
Chronic pancreatitis
Fig. 22.7 , p. 723 |
Irreversible glandular atrophy and dysfunction due to recurrent episodes of inflammation.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Calcifications within atrophic pancreas (i.e., fatty degeneration).
PP: Irregular dilation of the main pancreatic bile duct (best visible on thin-slice CT). May show hypodense thrombosis within otherwise hyperdense splenic vein or well-defined hypodense pancreas pseudocysts.
Diagnostic pearls: Irregularly dilated main pancreatic duct, glandular atrophy, pseudocysts, and tissue calcifications. |
Typically associated with alcohol abuse, but also observed in patients with recurrent cholelithiasis, pancreas divisum, or annular pancreas.
Tissue calcifications arise from reaction and precipitation of pancreas enzymes with Ca++ (from pancreatic tissue).
Often leads to glandular dysfunction with diabetes mellitus type II and fatty stool.
Pancreatic duct dilation due to pancreatic carcinoma occurs in 80% of cases confined to the pancreas head or body and appears less irregular.
Intraductal papillary mucinous neoplasm (IPMN) as another differential diagnosis may simulate chronic pancreatitis clinically and radiologically. |
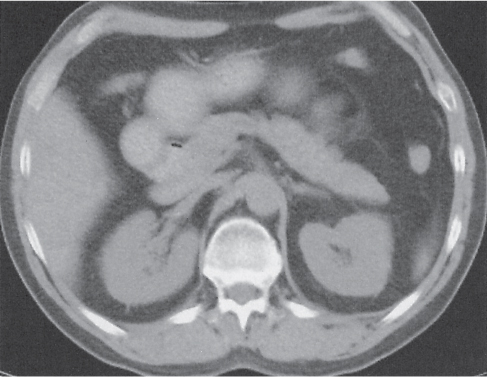
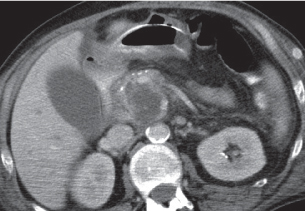
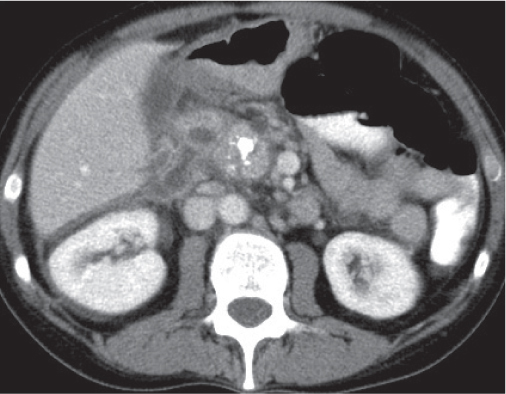
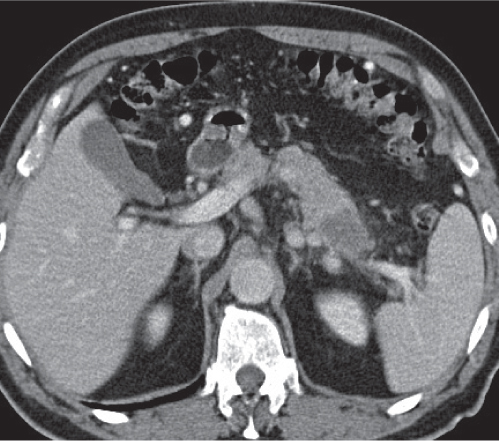
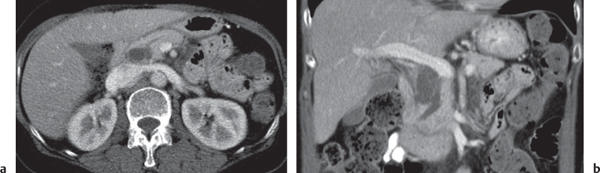
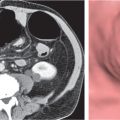
Pancreatic pseudocyst
Fig. 22.11 |
Fibrous encapsulated fluid collection of inflammatory pancreatic exudate.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Well-defined hypodense omental or retroperitoneal cystic lesion. Low-density intracystic gas bubbles seen in secondary infection. Cyst walls may calcify.
PP: Rim attenuation of pseudocyst.
Diagnostic pearls: Rim-enhancing, low-attenuating fluid collection in a patient with signs of chronic pancreatitis or following pancreas surgery/interventions. |
Histologically, a collection of necrotic tissue, blood, and enzymatic pancreatic fluid, encapsulated by granulation tissue and later a fibrous wall. During maturation, the density of the contents may decrease as blood and debris liquefy. May remain within the pancreatic capsule, but is more common in extrapancreatic locations such as the peritoneal cavity, retroperitoneum, or even mediastinum. Observed in about 10% of patients with pancreatitis. Nearly 50% resolve spontaneously within 6 weeks; the rest require drainage.
May simulate a necrotic or cystic tumor, pseudoaneurysm, or abscess. |
Cystic fibrosis (CF) |
Abnormal glandular function with lipomatous hypertrophy of the pancreas.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Hypodense aspect of whole pancreas with or without several nodular cysts and calcifications.
PP: Inhomogeneous, predominantly low-density attenuation (fatty infiltration) of the pancreas.
Diagnostic pearls: Fatty degeneration of the pancreas with multiple nodular cyst and scattered calcifications. |
CF is a recessively inherited multiorgan disease affecting particularly the lung, exocrine glands, and gut. Disorders of the pancreas are observed in > 80% of patients.
CF is the major reason for an insufficient exocrine pancreatic function during childhood.
Treatment through oral replacement of pancreas enzymes. |
Benign neoplasms |
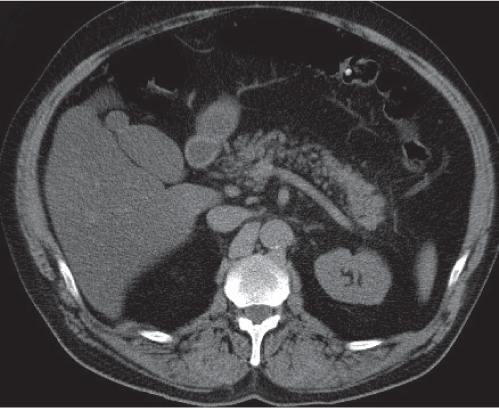
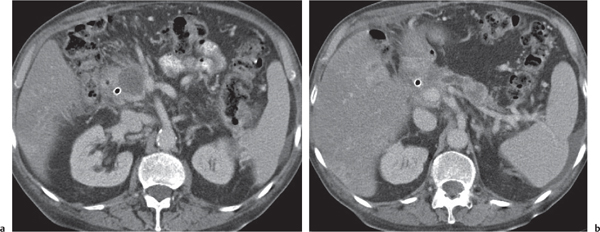
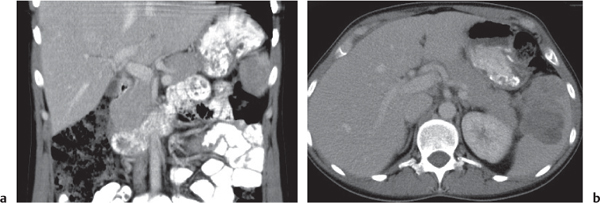
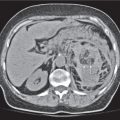
Serous cystadenoma (or microcystic) tumor
Fig. 22.12 a, b |
Cystic neoplasm, either macrocystic (cysts > 5 cm) or microcystic (cysts < 5 mm).
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Hypodense cysts. Calcifications may occur within a central scar.
PP: Honeycomb pattern of microcystic adenoma with septal and capsular contrast enhancement. Macrocystic adenoma remains hypodense without enhancement.
Diagnostic pearls: Well-defined, spongelike cystic mass in the head of the pancreas with distinct capsular/septal enhancement and normal width of main pancreatic duct (MPD). |
Rare (~1% of pancreatic neoplasms). Histologically, a glycogen-rich mucinous tumor arising from centroacinar cells.
Preferred location is the pancreas head, but it may occur in any part of the pancreas.
Simple cysts of the pancreas may be multiple (e.g., von Hippel–Lindau disease, polycystic disease) but typically have no internal architecture.
Differential diagnoses: IPMN, cystic islet cell tumor, and cystadenocarcinoma.
Serous cystadenoma has no malignant potential, but differentiation from these other cystic neoplasms based on imaging alone is often impossible. |
Pancreatic cyst |
Usually congenital true cysts that are caused neither by neoplastic nor by inflammatory disease.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Well-defined, round to oval, hypoattenuating cysts.
PP: Lesions remain hypodense without enhancement. |
Histologic true cysts with epithelial lining.
Typically associated with cystic disease of the liver and other organs, such as autosomal dominant polycystic kidney disease (ADPKD) or von Hippel–Lindau disease.
Solitary or multiple collection of near-water density is seen within the pancreas. |
Malignant neoplasms |
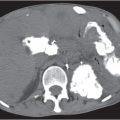
Cystadenocarcinoma, mucinous cystic pancreatic tumor
Fig. 22.13 |
Cystic neoplasm of varying size (3–10 cm) in the body or tail of the pancreas.
Scan recommendation:
Multiphasic CT (nonenhanced CT, portal venous phase [PVP], PP).
Nonenhanced CT: Hypodense unilocular cystic mass with or without calcifications.
PVP: Small tumors may be better delineated during an early contrast phase.
PP: Distinct septal/capsular contrast enhancement. Macrocystic adenoma remains hypodense without enhancement.
Diagnostic pearls: Unilocular cystic mass in the body or tail of the pancreas with hyperattenuation of thick, irregular wall on postcontrast scans. |
Cystic malignant neoplasms represent < 10% of all malignant neoplasms. Cysts are typically located distal to the tumor, representing either pseudo- or retention cysts.
As compared with simple cysts or pseudocysts, malignant neoplasms usually show higher central attenuation values and have irregular, thicker walls. Carcinomas may be masked in the presence of chronic inflammation. Ductal dilation often is the only abnormality on CT scans. In > 50% of patients, pancreatic duct (5–10 mm) and biliary tract dilation is observed. Stranding of peripancreatic fat is indicative of lymphangiosis and thus suspicious for extraglandular tumor growth. Mesenteric lymph nodes may be < 10 mm but still be infiltrated. |
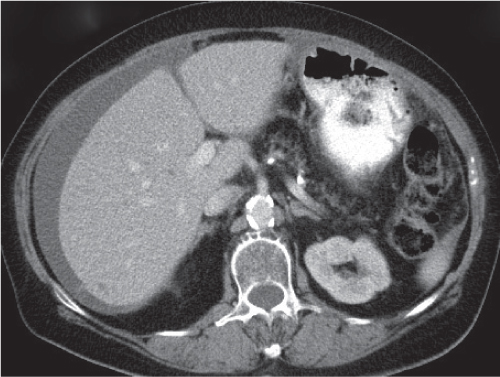
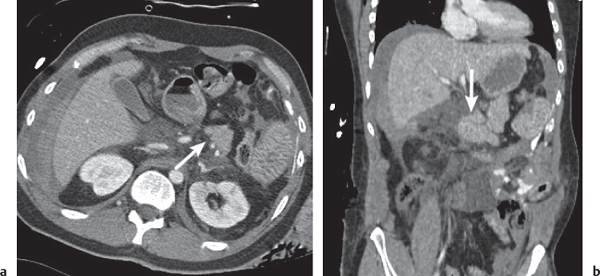
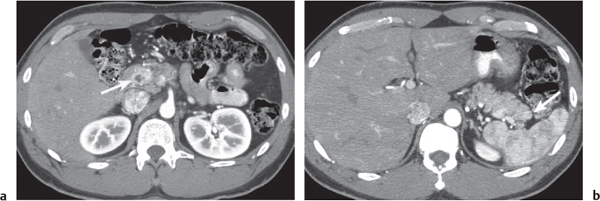
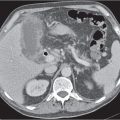
Ductal pancreas carcinoma
Fig. 22.14a, b |
Pancreas carcinoma arising from ductal epithelium of exocrine pancreas.
Scan recommendation:
Biphasic CT (nonenhanced CT, PP).
Nonenhanced CT: Hypo-/isodense to normal pancreas tissue.
PP: Heterogeneous, often poor enhancement.
Diagnostic pearls: Heterogeneous, poorly enhancing irregular mass in the pancreas head with dilated common bile duct (CBD) and MPD (double duct sign). |
Ductal pancreas carcinoma represents 95% of all pancreatic neoplasms. Histologically, presents as mainly mucinous adenocarcinomas.
Sixty percent in the head, 20% in the body, 5% in the tail, and 15% diffuse.
Grading into three stages:
I: confined to pancreas
II: stage I and regional lymph node metastases
III: stage II and distant metastases |
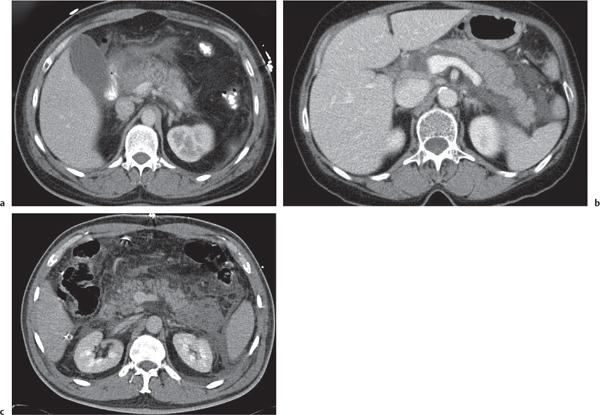
Islet cell tumor
Fig. 22.15a, b |
Neuroendocrine tumors arising from endocrine pancreatic cells (islet cells of Langerhans).
Scan recommendation:
Multiphasic CT (nonenhanced CT, arterial phase [AP], PVP). Nonenhanced CT: Small or large lesion, isodense to normal pancreatic tissue. Calcifications may be present.
AP: Small islet cell tumors enhance usually strong and homogeneously.
Large tumors may show ring enhancement with central necrotic low-density area.
PVP: Tumors quickly become isodense (masked) to normal pancreas.
Diagnostic pearls: A small hypervascular pancreatic mass with several liver lesions, showing a similar enhancement pattern. |
Islet cell tumors are either secretory (functioning) or nonfunctioning. Secretory tumors are detected earlier due to clinical symptoms. Nonfunctioning tumors are usually large at the time of detection. Sixty to 75% of secretory islet cell tumors secrete insulin (insulinomas), and 20% are gastrin-secreting alpha-1 islet cell tumors (gastrinomas), causing Zollinger–Ellison syndrome.
Rare islet cell tumors include those producing glucagon (alpha-2 cell), vasoactive intestinal peptide (VIPoma, non-beta cells), and somatostatin (somatostatinoma [delta cells]). Amine precursor uptake and decarboxylation cell tumors (APUDomas) are occasionally encountered in the pancreas. They produce adrenocorticotropic hormone (ACTH), antidiuretic hormone (ADH), and vasoactive intestinal polypeptide (VIP).
Liver metastases are often detected before diagnosis of the primary islet cell tumor. |
Papillary cystic carcinoma, solid and papillary tumor of the pancreas
Fig. 22.16 |
Well-defined large (> 2 cm) heterogeneous mass in tail or body of the pancreas.
Scan recommendation:
Biphasic CT (nonenhanced CT, PVP).
Nonenhanced CT: Well-defined, centrally hypodense mass, depending on hemorrhage/necrosis ratio. Calcifications may be present.
PVP: Lesion remains centrally hypoattenuating with thick and strongly enhancing wall.
Diagnostic pearls: Well-defined large (> 2–20 cm) tumor in the body or tail of the pancreas with coexistence of solid and cystic components. |
Rare. Histologic solid and pseudopapillary structures surrounded by areas of necrosis and hemorrhage. Typically occurs in young (< 40 y) patients.
Surgery is the treatment of choice.
Rarely recurs after excision; thus, overall prognosis is good. |
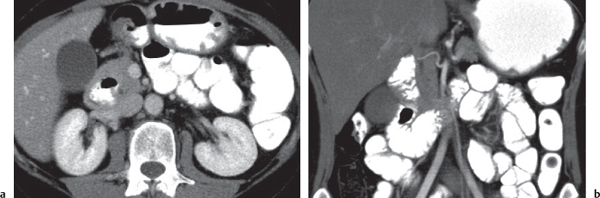
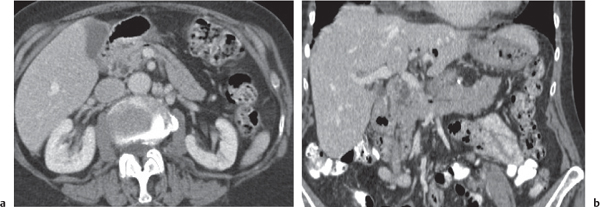
Intraductal papillary mucinous neoplasm (IPMN)
Fig. 22.17a, b |
Mucin-producing neoplasm arising from the epithelial lining of the MPD and/or branch pancreatic ducts (BPD type).
Scan recommendation:
Biphasic CT (nonenhanced CT, PVP).
Nonenhanced CT: Dilated and tortuous main duct in MPD type. Rarely calcifications.
Nonenhanced CT: Dilated and tortuous main duct in MPD type. Rarely calcifications.
Grapelike cluster of multiple small hypodense cysts in BPD type.
PVP: Subtle, irregular enhancement of cyst wall lining.
Diagnostic pearls: Grapelike, hypoattenuating cluster of micronodular cysts within the uncinate process and emptying into a dilated MPD. |
MPD IPMN usually located in body or tail, BPD type usually in the pancreatic head and uncinate process. Thirty percent of tumors transform into malignant variant.
Usually diagnosed in patients older than 60 y. Symptomatic IPMN may cause pain, recurrent pancreatitis, diabetes mellitus, diarrhea, and weight loss.
Treatment of choice is continuous monitoring in old and asymptomatic patients and surgical resection in young and symptomatic patients. |
Pancreatic metastases |
Pancreatic lesions without obstruction of the MPD.
Scan recommendation:
Biphasic CT (nonenhanced CT, PVP).
Nonenhanced CT: Usually isodense to normal pancreas (melanoma: hyperdense).
PVP: Variable; may resemble primary pancreas tumor.
Diagnostic pearls: Intrapancreatic lesion (s) with concomitant intra-abdominal metastases (lymph nodes, adrenal glands, kidneys, liver). |
Most often metastases are from melanoma, lung cancer, breast cancer, and ovarian cancer. Carcinoma of the stomach, gallbladder, and liver may directly invade pancreatic tissue.
Lesions of the left adrenal gland and kidney initially displace the tail of the pancreas, subsequently invade peripancreatic fat, and may finally occlude the splenic vein. |
Lymphoma
Fig. 22.18a, b |
Either a large, homogeneous, solid intraparenchymal mass or enlarged peripancreatic lymph nodes.
Scan recommendation:
Biphasic CT (nonenhanced CT, PVP).
Nonenhanced CT: Diffuse pancreas enlargement with stranding of perihepatic fat, enlarged peripancreatic lymph nodes.
PVP: Usually isoattenuating to pancreas; sometimes subtle enhancement of tumor.
Diagnostic pearls: Enlarged peripancreatic lymph nodes or diffusely enlarged pancreas in a patient with known lymphoma. |
Primary lymphoma represents < 1% of all pancreatic neoplasms. Secondary lymphomas are more common. Most common subtype is non–Hodgkin lymphoma. Treatment of choice usually is chemotherapy. |