Vascular |
Atherosclerosis
Fig. 18.35a, b , p. 653 |
Fatty deposits (atheromas) inside the arterial walls leading to stenosis and calcification of the arteries.
Diagnostic pearls: Vessel tortuosity, intimal calcification, noncalcified atheromatous plaques, and sometimes thrombus formation. |
Atherosclerotic risk factors (see Framingham risk factors) initiate vascular stress with subsequent local vascular inflammation, progressive intimal thickening, and finally stenosis. May also lead to formation of an intravasal thrombus or an aneurysm. Plaques may be calcified (stable) or noncalcified (more prone to rupture) (e.g., atheromas). Eccentric array of calcifications is an important differentiator to aortic dissection (centrally displaced intima). |
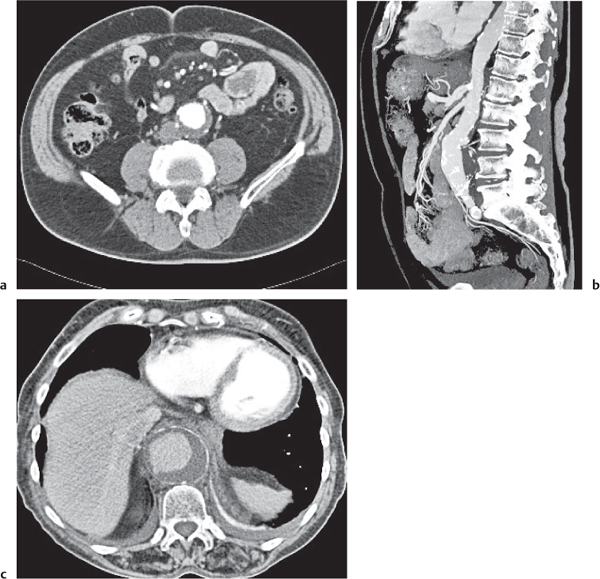
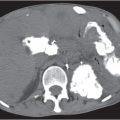
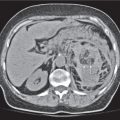
Aortic aneurysm
Fig. 26.2a–c |
Segmental to total saccular or fusiform aortic dilation < 3 cm in diameter.
Diagnostic pearls: Thickened, often calcified, a ortic wall; calcifications found on outer wall of dilated aorta; presence of noncalcified plaques/thrombus. Perianeurysmal fat stranding indicates inflammatory fibrosis. Discontinuity of the aneurysm wall and periaortic hematoma are indicative of rupture. |
Observed in 1% to 3% of the Western population, mainly due to atherosclerosis.
Crawford classification for staging:
Type 1: Aneurysm from left subclavian artery to renal arteries.
Type 2: Aneurysm from left subclavian artery to aortic bifurcation.
Type 3: Aneurysm from middescending aorta to aortic bifurcation.
Type 4: Aneurysm from upper abdominal aorta and all or none of the infrarenal aorta.
The risk of rupture increases with aneurysm diameter. Surgical/interventional repair required for > 1 cm/y diameter growth rate or an aneurysm size > 5 cm. Differential diagnosis: Intramural hematoma (IMH)/aortic dissection. |
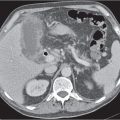
Aortic dissection
Fig. 18.40a–c , p. 655 |
Spontaneous intimal tear with continuous mucosal wall separation.
Diagnostic pearls: On nonenhanced CT, calcified intima are displaced centrally. On contrast-enhanced CT (arterial phase), contrast-filled double (true and false lumen) channel with an intervening intimal flap spirals down the aorta.
Indicators of the false lumen:
• Usually the larger lumen with delayed contrast
• A “beak” sign: An acute angle between the dissected flap and the outer wall
• Presence of cobwebs: Thin mucosal strands crossing the false lumen
• Intraluminal thrombus with centrally displaced intima (calcifications). In general, eccentric array of calcifications is an important differentiator to aortic dissection (centrally displaced intima).
Characterized by a contrast-filled double channel with an intervening intimal flap. True and false lumen usually opacify at different paces, the true lumen first. I ntraluminal thrombosis, dilation of the aorta with compression of the true lumen, and irregular contour of the contrast-filled part of the aorta are other common findings. |
Histologically, a cystic media necrosis from either a therosclerosis or congenital disorders.
May be due to collagen disorders (Ehlers–Danlos syndrome, Marfan syndrome, Turner syndrome, osteogenesis imperfecta, etc.), trauma, hypertension, or pregnancy (> 50% of dissections in women).
Stanford classification of dissections:
Type A: Entry of dissection in ascending aorta (70%)
Type B: Entry of distal to left subclavian artery (30%) Intimo-intimo intussusception is observed in cases of complete circumferential dissection.
Typical complications include occlusion of the aortic side branches and continuation into the iliac arteries. Multiplanar reconstruction (MPR) is useful for assessment of exact dimension of dissection.
Look out for distal re-entry.
Typical pitfalls are streak (i.e., motion artifacts in the ascending aorta). When in doubt, perform electrocardiogram (ECG)-triggered CT. |
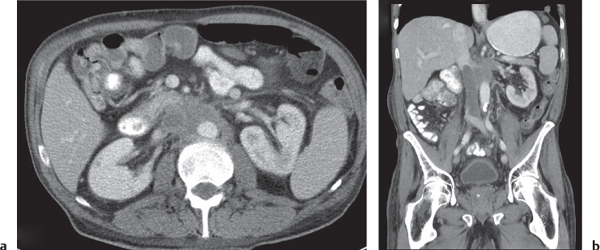
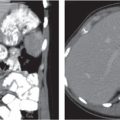
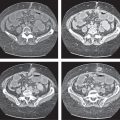
Aortic surgery
Fig. 26.3a, b |
History of previous interventional stent or surgical graft placement.
Diagnostic pearls: Any high-attenuating homogeneous perigraft mass with decreasing HU values > 2 weeks after surgery suggests hematoma. Fluid–fluid levels indicate sedation of hemoglobin.
Irregular, septated periaortic fluid or gas collection is indicative of an infected graft.
Irregular, periaortic fat stranding on nonenhanced CT and moderate attenuation on postcontrast scans is typical of stent/graft infection.
Septated periaortic fluid or gas collection is highly suspicious of abscess formation.
The lack of luminal contrast enhancement is observed in thrombosis.
A blood-equivalent mass lesion with rim enhancement and with or without septations may represent a pseudoaneurysm. |
Aortic graft may be end-to-side, end-to-end, or intra-aneurysmal.
Grafts may extend into the iliac arteries (Y graft).
Ventral collections of perigraft gas and fluid shortly after surgery may be a normal postoperative finding. Differential diagnoses: seroma and lymphocele.
A common complication of endovascular aneurysm repair (EVAR) represent endoleaks, which may continue to perfuse and pressurize the aneurysm sac, thereby conferring an ongoing risk of aneurysm enlargement and/or rupture.
Endoleaks are classified by the source of blood flow and organized into five categories:
I: Attachment site leaks
II: Collateral vessel leaks
III: Graft failure (i.e., midgraft hole, junctional leak, or disconnect)
IV: Graft wall porosity
V: Endotension (with or without endoleak) |
Circumaortic left renal vein |
Congenital anomaly of the left renal vein.
Diagnostic pearls: One of two left renal veins crosses the aorta anteriorly and posteriorly to join the IVC. |
Incidence: 1.5% to 8.7%. |
Retroaortic left renal vein |
Diagnostic pearls: One left renal vein crosses the aorta posteriorly. |
Incidence: 2% to 2.5%. In 0.1% of cases, a concomitant retrocaval ureter is seen. |
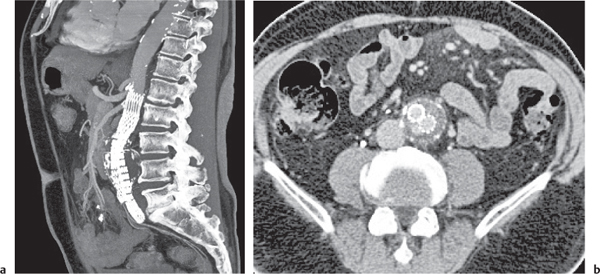
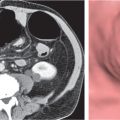
Caval transposition/left inferior vena cava (IVC)
Fig. 26.4 |
Congenital anomaly of the IVC inferior to renal veins.
Diagnostic pearls: On caudal sections, the IVC parallels the aorta to the left and ends at the level of the left renal vein, which crosses the aorta either anteriorly or posteriorly to form a normal right suprarenal IVC. |
Incidence: 0.2% to 0.5%. |
Duplication of the inferior vena cava (IVC) |
Persistence of both supracardinal veins.
Diagnostic pearls: Left and right IVC inferior to the renal vein. Left IVC drains into the left renal vein, which crosses the aorta in normal fashion and joins the IVC. May be combined with an azygos/hemiazygos continuation of the IVC. |
Incidence: 0.2% to 3.0%. A duplicated IVC may mimic a prominent left gonadal vein.
The latter can be traced to the level of the inguinal canal or ovary, whereas a duplicated IVC ends at the level of the left common iliac vein. |
Azygos continuation of the inferior vena cava (IVC) |
Failure to form right subcardinal-hepatic anastomosis, resulting in atrophy of the right subcardinal vein.
Diagnostic pearls: Intrahepatic vena cava is absent, and the IVC passes the diaphragm posteriorly as the azygos vein, which joins the superior vena cava (SVC) at the physiological location in the right peribronchial location. |
Incidence: 0.2% to 4.3%.
It is associated in 85% of cases with congenital heart disease; left pulmonary isomerism, polysplenia, dextrocardia, and intracardiac defects.
Transposed abdominal viscera are also commonly associated. |
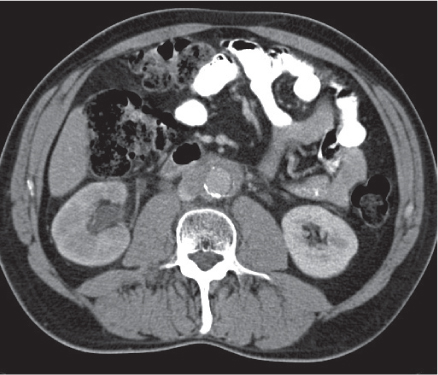
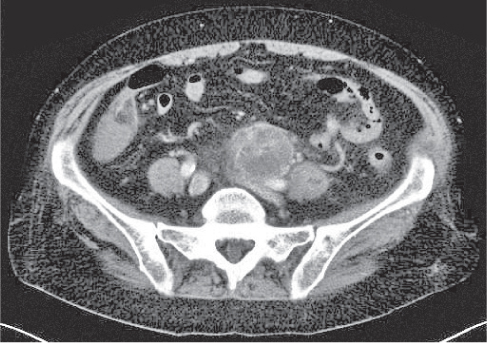
Thrombosis of the inferior vena cava (IVC)
Fig. 26.5a, b |
Diagnostic pearls: A low-density intraluminal filling defect in a focal enlarged IVC, often surrounded by a rim of high density representing the vessel wall.
The presence of gas bubbles within the caval filling defect indicates septic thrombosis. |
May result from bland or septic thrombosis, but is more commonly secondary to direct tumor invasion from the kidney, adrenals, or liver. |
Inflammation |
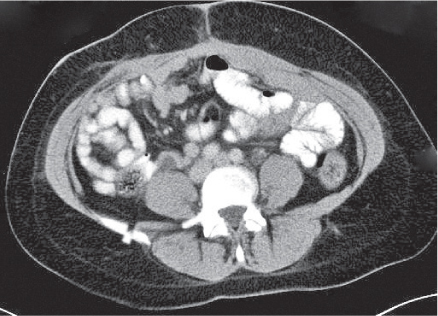
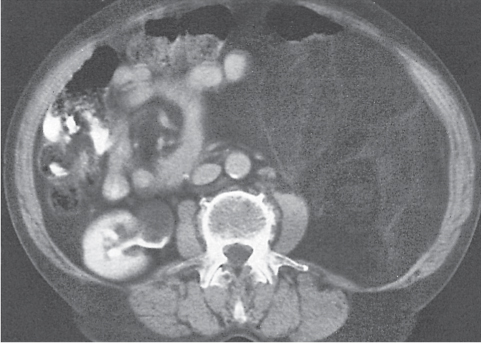
Retroperitoneal fibrosis
Fig. 26.6 |
Thick, fibrous soft tissue encasing retroperitoneal vessels.
Diagnostic pearls: A distinctly enhancing, fibrous sheet/bulky mass extending from the kidneys to the sacrum. Also seen: encasing of the aorta, IVC, iliac vessels, and ureters. Retroperitoneal fibrosis usually displaces the ureters medially, unlike retroperitoneal lymphadenopathy, which tends to displace them laterally. |
Proliferation of fibrous and inflammatory tissue in the retroperitoneum. Seventy percent of cases are idiopathic; 30% are secondary to either drugs (methysergide or ergotamine) or malignant tumors, which cause a desmoplastic reaction that is morphologically indistinguishable from retroperitoneal fibrosis. |
Sarcoidosis |
Lymphadenopathy associated with hepatosplenomegaly in the absence of signs of portal hypertension.
Diagnostic pearls: Discrete lymph node swelling, unlike in malignant lymphoma. |
Histologically, well-defined granulomas with a rim consisting of fibroblasts and lymphocytes.
Usually associated with chronic pulmonary sarcoidosis (75%). Differentiation from lymphoma may be difficult without biopsy. |
Amyloidosis |
Lymphoma-like lymph node enlargement.
Diagnostic pearls: Nonspecific hepatomegaly and lymphadenopathy.
Mural involvement of gastrointestinal (GI) organs is more common. |
Deposition of protein-polysaccharide material in various organs. Can be either primary or secondary to advanced age, rheumatoid arthritis, multiple myeloma, lymphoreticular malignancy, or chronic infections. |
Whipple disease |
Bacterial infection leading to malabsorption and chronic diarrhea.
Diagnostic pearls: Lymphadenopathy with central areas of low density, caused by deposition of fat and fatty acids. |
Characteristic of Whipple disease but may also occur in Crohn disease, treated lymphoma, and metastases. |
Castleman disease |
Rare benign lymphoproliferative hyperplasia of the lymph nodes.
Diagnostic pearls: Well-defined muscle density mass with a thin, enhancing rim that may contain spotty central calcifications. |
Histologically, hyaline vascular (90%), plasma cell (9%), or mixed types.
Observed in middle-aged adults without any gender preponderance.
Castleman disease is indistinguishable from lymphoma. Seventy percent of the lesions occur in the middle or posterior mediastinum, rarely in the retroperitoneum. |
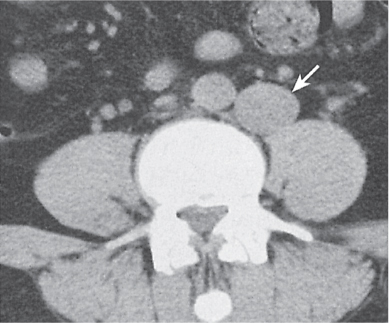
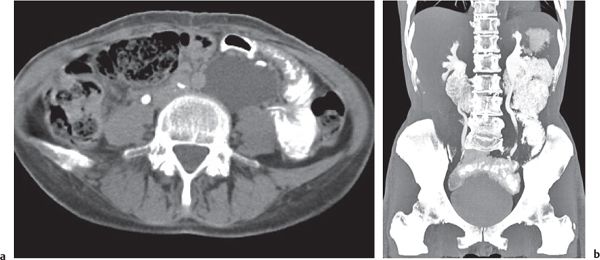
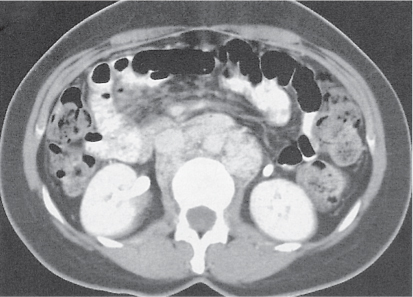
Benign lymphadenopathy
Fig. 26.7 |
Diagnostic pearls: Reactively enlarged lymph nodes up to 2 cm, especially in immunosuppressed patients. |
Relatively rare. In immunocompromised patients, it is impossible to distinguish between lymphomas and metastases of Kaposi sarcoma. |
Congenital |
Undescended testes |
Diagnostic pearls: Small retroperitoneal focus of soft tissue anywhere along the course of the gonadal vein from the inguinal region to the renal vein. Growth indicates malignancy. |
Eighty percent of maldescended testes are located distal to the inguinal ring, 20% are above and usually adjacent to the iliac vessels.
Congenital absence of testes occurs. |
Trauma |
Hematoma |
Irregular soft tissue mass that obscures, compresses, or distorts normal retroperitoneal structures.
Diagnostic pearls: During acute stage, hematomas are high-attenuating.
Subacute hematomas appear heterogeneous with higher density centrally and a more lucent periphery (due to liquefaction). Chronic hematomas may develop a thick calcified capsule. |
Either spontaneous or secondary to trauma, vascular tumors, aneurysm rupture, blood dyscrasias, anticoagulation therapy, or long-term hemodialysis. Usually contained within well-defined muscle groups and spontaneously resolving. |
Infection |
Tuberculosis (TB) |
Diagnostic pearls: Lymphadenopathy in mesenteric and peripancreatic lymph nodes. Central areas of low attenuation and inflammatory rim enhancement following intravenous (IV) contrast administration are typical, but they may also be seen in metastatic nodes. |
Complicates pulmonary disease in 6% to 38% of immunocompromised patients.
May be due to Mycobacterium tuberculosis or M. avium intracellulare (MAI). The latter typically shows lymph nodes with distinct central necrosis. |
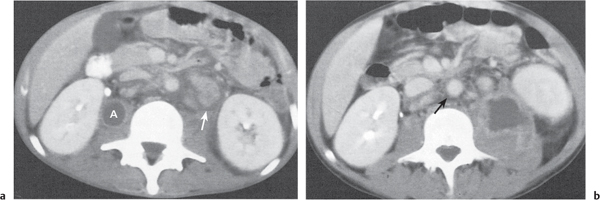
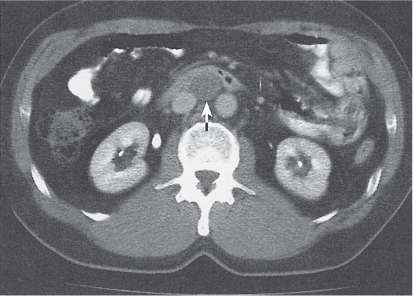
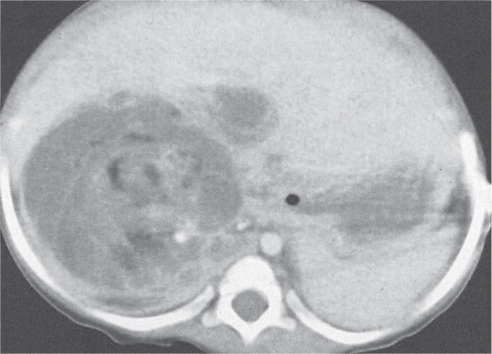
Abscess
Fig. 26.8a, b |
Diagnostic pearls: Well-defined mass of fluid density with or without gas bubbles. Usually strong rim enhancement. |
The most common causes are colonic perforation, pancreatic abscess, duodenal perforation, and surgical contamination. Very common in immunocompromised patients. |
Benign neoplasms |
Lymphocele |
Homogeneous, low-attenuating fluid collection due to iatrogenic disruption of lymphatics.
Diagnostic pearls: Smoothly marginated (very thin), waterlike, hypodense lesion. Secondary infection may result in higher attenuation values. |
Typically occurs several weeks after retroperitoneal surgery or intervention. |
Urinoma (perirenal pseudocyst) |
Water-dense mass that partially or completely occludes the perirenal compartment.
Diagnostic pearls: In acute stages, leakage of contrast material into the urinoma may be visible. Chronic urinoma may extend down to the pelvic inlet. |
Ill-definition from adjacent structures or attenuation values > 30 HU is suspicious of an infection.
A thick, enhancing rim is typically observed in abscess formation. |
Seroma |
Diagnostic pearls: Collection of near-water-density fluid, usually in a postoperative patient at or near the site of operation. |
May become infected and develop into an abscess. |
Cystic lymphangioma |
Diagnostic pearls: Solitary or multilocular cystic mass with slightly enhancing, thin walls. |
Neoplasms that almost exclusively occur in the neck or groin. Very rarely found in the retroperitoneum. |
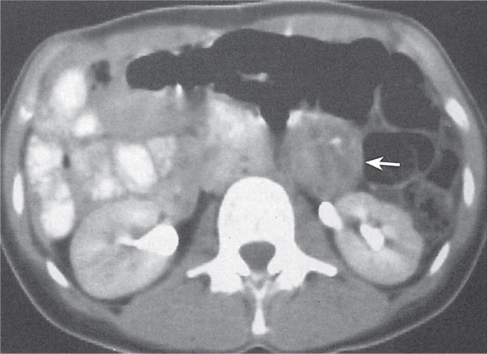
Pancreatic pseudocyst
Fig. 22.11 , p. 725 |
Fibrous encapsulated fluid collection of inflammatory pancreatic exudate.
Diagnostic pearls: Near-water-density mass with enhancing walls. |
May invade the retroperitoneal space but usually stays outside Gerota fascia. |
Malignant neoplasms |
Lymphoma
Fig. 26.9a, b
Fig. 26.10 |
Diagnostic pearls: Well-defined enlarged lymph nodes that may coalesce into large conglomerates. Typically results in arterial displacement of vessels and ureters. |
CT does not provide any information concerning the type of lymphoma. It can only detect enlarged lymph nodes. |
|
Attenuation of enlarged lymph nodes is similar to that of muscle tissue (40–60 HU). |
Staging according to Ann Arbor classification (I–IV):
Involvement of only one (stage I) or two (stage II) nodal regions on one side of the diaphragm has a better prognosis than involvement of nodal regions on both sides of the diaphragm with or without involvement of extralymphatic organs (stages III and IV). |
Testicular neoplasm
Fig. 26.11 |
The most common malignancy in men age 20 to 34 y.
Diagnostic pearls: Enlarged, low-attenuation, para-aortic lymph nodes near the renal hilum. |
About 95% of neoplasms are germ cell tumors, including seminoma, embryonal cell tumor, teratocarcinoma, choriocarcinoma, and mixed-element tumors. |
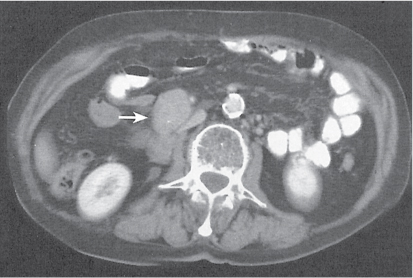
Other metastatic tumors
Fig. 26.12 |
Enlarged retroperitoneal lymph nodes in the pelvic and abdominal regions.
Diagnostic pearls: Any round lymph node > 1 cm and without central fat hilus is suspect.
Metastatic lymph node enlargement is less pronounced than in malignant lymphoma.
Metastatic nodes may not be enlarged. |
Cervical, prostatic, bladder, uterine, renal, and ovarian carcinomas frequently metastasize to retroperitoneal lymph nodes.
Other primaries include lung and GI carcinomas. Modest lymph node enlargement in renal cell carcinoma patients may be reactive, not metastatic. |
Mesodermal tumors
Fig. 26.13
Fig. 26.14
Fig. 26.15 |
Large mass that may be solid (soft tissue density), mixed, or pseudocystic (water-like density).
Diagnostic pearls: CT attenuation depends on the main soft tissue component.
A leiomyosarcoma is strongly enhancing with a central necrosis.
A liposarcoma has higher attenuation than normal retroperitoneal fat and shows large soft tissue components. The lack of soft tissue suggests a benign lipoma. |
Fifty percent of all mesodermal tumors are primary retroperitoneal neoplasms, of which 85% are malignant. Liposarcoma is the most common type. Even a subtle area of negative CT values in a solid retroperitoneal mass is highly suspicious of liposarcoma. Other tumor entities are leiomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma, malignant hemangiopericytoma, and malignant mesenchymoma. |
Neurogenic tumors
Fig. 26.16 |
Small, well-defined, round to oval retroperitoneal mass.
Diagnostic pearls: Hypodense masses near the course of a nerve. |
Neurogenic tumors represent 30% of primary retroperitoneal tumors. Usually occur in patients younger than 30 y.
Ganglioneuroma, pheochromocytoma, and neurofibroma are the most common benign tumors. Neuroblastoma is a common malignancy in children younger than 6 y. Patients with von Hippel–Lindau syndrome, tuberous sclerosis, or neurofibromatosis have a genetic predisposition for neurogenic tumors. |