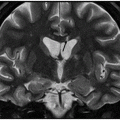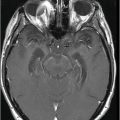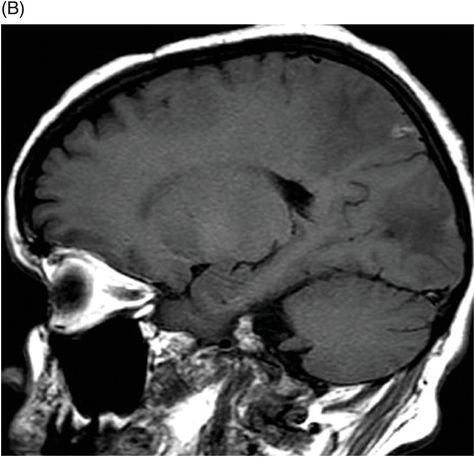
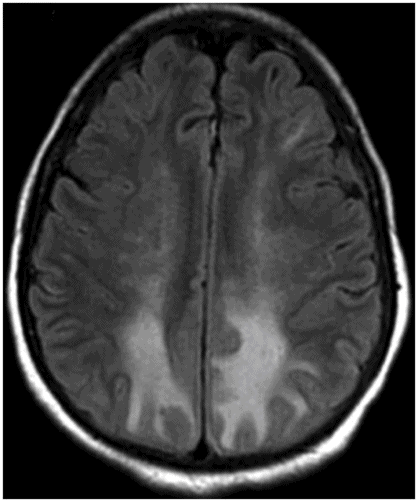
Axial FLAIR image through the centrum semiovale.
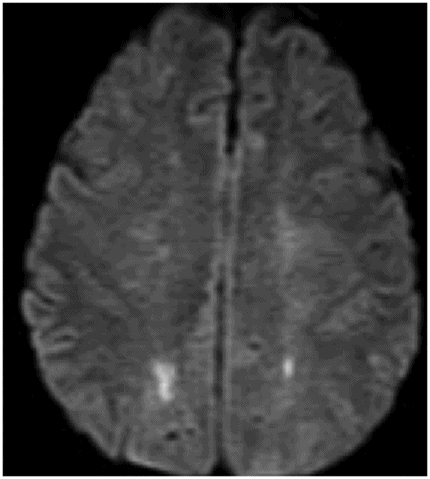
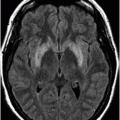
Axial DWI image through the centrum semiovale.
Cerebral Amyloid Angiopathy with Inflammation
Primary Diagnosis
Cerebral amyloid angiopathy with inflammation
Differential Diagnoses
Cerebral amyloid angiopathy
Primary angiitis of CNS
Imaging Findings
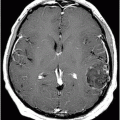
Fig. 24.1: (A) Axial T2*-weighted sequence through the level of the centrum semiovale demonstrated multiple foci of microhemorrhages in the cerebral cortices in bilateral posterior parietal lobes and in the left frontal lobe. (B) Sagittal left paramedian T1WI demonstrated tiny foci of hemorrhage in the posterior parietal regions and hypointensity in the adjacent brain parenchyma. Fig. 24.2: Abnormal hypointensity can also be noted in the left occipital lobe gray-white interface. Axial FLAIR image through the centrum semiovale demonstrated abnormal FLAIR hyperintensity involving bilateral posterior parietal gray-white interface suggestive of brain edema. Subtle FLAIR hyperintensity was also noted in the left frontal subcortical white matter as well, in the region of the microhemorrhages. Increased FLAIR signal was also noted involving left occipital lobes as well (not shown). Fig. 24.3: Axial DWI image through the centrum semiovale demonstrated punctate areas of diffusion restriction (with low value on ADC map, not shown) suggestive of punctate areas of infarction involving bilateral posterior parietal lobes.
Discussion
The presence of cortical microhemorrhages in an elderly patient is highly suggestive of cerebral amyloid angiopathy (CAA). However, edema in the adjacent brain tissue is not a common finding in a patient with CAA in the absence of inflammation or tiny infarcts. Subacute-onset neurologic symptoms, cortical microhemorrhages, tiny infarcts, and extensive edema in the adjacent brain parenchyma is highly suggestive of cerebral amyloid angiopathy with inflammation. Autoantibodies against Aβ 1–40 in the spinal fluid further support the diagnosis of amyloid β-related angiitis (ABRA), one of the cerebral amyloid angiopathy with inflammation variants, which was confirmed on biopsy.
Cerebral amyloid angiopathy-related inflammation (CAA-RI), another variant of cerebral amyloid angiopathy with inflammation, may have similar clinical presentation and CSF findings, but the presence of hemorrhage and infarcts are not common. Cerebral amyloid angiopathy without any inflammation more commonly has neurocognitive decline and frank intraparenchymal hemorrhages, compared to the two variants of cerebral amyloid angiopathy with inflammation. In addition, CAA presents at a slightly higher age group and usually has numerous microhemorrhages involving extensive areas of superficial cortical tissue rather than a few foci of microhemorrhages, as seen in this patient. Primary angiitis of CNS (PACNS) shares many of the imaging abnormalities with cerebral amyloid angiopathy with inflammation. However, clinical characteristics are different. Primary angiitis of CNS is primarily a disease of much younger patients who typically present with multifocal infarction, headache, and focal neurologic deficit. Intracranial hemorrhage is rare. Neurocognitive decline is not a dominant clinical finding of PACNS (please see Part IV: Case 51, for in-depth discussion of PACNS).
Sporadic CAA is deposition of Aβ in the wall of the leptomeningeal and cortical vessels. It weakens the vessel wall and results in vessel fragility, rupture, and intraparenchymal hemorrhage. Hemorrhage can extend to the adjacent subarachnoid component. As such, this sporadic form of CAA is not associated with any vessel wall inflammation. However, some patients with CAA do demonstrate Aβ deposition-associated inflammation. Two distinct subtypes have been identified: one with perivascular inflammation, known as CAA-RI, and a more common, destructive, transmural vasculitic process, ABRA. Owing to extensive transmural vasculitis, it has been proposed that ABRA is more closely related to PACNS than CAA. Amyloid β-related angiitis is characterized by severe leptomeningeal and cortical amyloid angiopathy and mild to moderate chronic inflammatory infiltrates in the adjacent brain parenchyma that is often granulomatous. Splitting of vessel walls, fibrinoid necrosis, acute thrombosis, and chronic thrombosis with recanalization are common histopathologic findings. Quantitative immunohistochemical studies have shown that ABRA is associated with excessive immune activation against Aβ with associated enhanced clearing of the Aβ from the adjacent brain parenchyma. Cognitive decline and headache are the most common clinical ABRA presentations. The most common imaging abnormalities seen in patients with ABRA are vasogenic edema and leptomeningeal enhancement. Intraparenchymal enhancement has also been described and infarction can be seen in up to 25% of patients. Intraparenchymal hemorrhage is less common in ABRA, as compared to CAA, 18% versus 60%.
A distinct clinicopathologic entity, ABRA differs from CAA in multiple ways. Patients with ABRA are younger than CAA at the time of diagnosis, and have lower frequency of neurocognitive decline and intraparenchymal hemorrhage. Leptomeningeal and intraparenchymal enhancement is more common in ABRA. It responds well to steroids, suggesting that the key underlying pathology is an inflammatory process with favorable prognosis, whereas CAA is a progressive disease and is considered untreatable.
Significantly different from PACNS patients, ABRA patients are older than PACNS patients at the time of diagnosis and have an increased frequency of altered cognition and seizures. Leptomeningeal enhancement is a more common neuroimaging abnormality in ABRA, as compared to PACNS, in which infarction is a dominant feature. The response to treatment and overall prognosis is similar in both ABRA and PACNS.
Autoantibodies against Aβ 1–40 and 1–42 have been associated with ABRA and CAA-RI, further suggesting an underlying inflammatory role in these two conditions. In fact, treatments targeting amyloid load reduction and monoclonal antibody against Aβ in Alzheimer disease have been shown to simulate clinical and imaging abnormalities like that of ABRA and CAA-RI. In addition, both the spontaneous amyloid-related inflammatory conditions and drug-induced inflammatory conditions share a common genetic associated with the APOE ε4 allele.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree