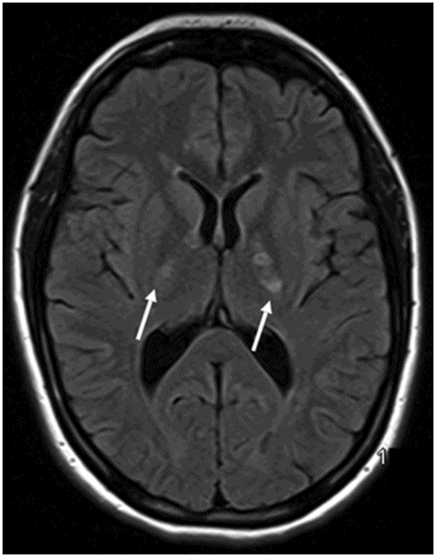
Axial FLAIR image at the level of the centrum semiovale.
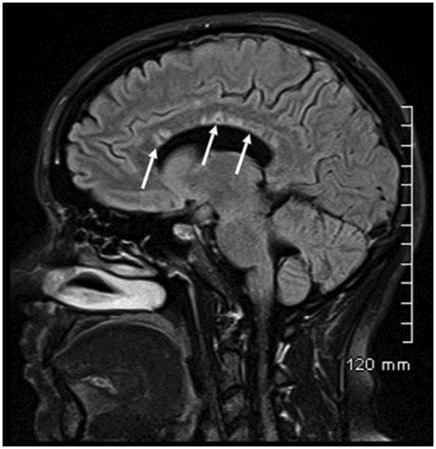
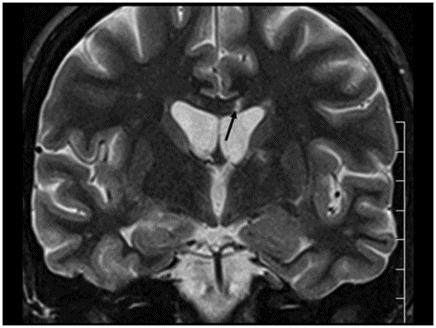
Sagittal FLAIR image near the midline.
Susac Syndrome
Primary Diagnosis
Susac syndrome
Differential Diagnoses
Multiple sclerosis
Encephalitis
Acute disseminated encephalomyelitis (ADEM)
Imaging Findings
Fig. 26.1: Axial FLAIR image at the level of the centrum semiovale demonstrated small hyperintense foci in the deep white matter, without mass effect. Note that some of the lesions are rounded in shape and have a centralized low signal (following the CSF), surrounded by a hyperintense rim. These findings likely represent chronic lacunar infarcts surrounded by gliotic tissue (arrows). Diffusion-weighted imaging and postcontrast T1WI (not shown here) were unremarkable. Fig. 26.2: Axial FLAIR image at the level of the basal ganglia showed small rounded hyperintense lesions in the posterior limbs of the external capsules, particularly on the left side, with similar appearance to the ones in the centrum semiovale (arrows). The lesions on the left side are arranged in a linear array resembling a string-of-pearls. Fig. 26.3: Sagittal FLAIR image near the midline showed small rounded and irregular hyperintense lesions in the corpus callosum. Note the characteristic, round snowball-like lesions (arrows) with no significant mass effect. Fig. 26.4: Coronal T2WI at the level of the frontal horns demonstrating that the same lesions in the corpus callosum are sparing its outer margins (arrow).
Discussion
The triad of encephalopathy, and visual and hearing loss, when accompanied by characteristic imaging findings (white matter ischemic changes in the deep white matter of the cerebral hemispheres and the posterior limb of the internal capsules [with the string-of-pearls appearance], and in the central portions of the corpus callosum, with the typical snowball appearance), in association with abnormal retinal fluorescein angiography (RFA), is highly suggestive of Susac syndrome (SS).
Multiple sclerosis (MS) is a well-known entity that demonstrates white matter changes typically involving the corpus callosum (CC) and cerebral white matter. Patients with MS, moreover, often complain of visual abnormalities, reflecting optic nerve demyelination (optic neuritis), but not hearing loss. Classically, MS patients present with a typical remitting-relapsing clinical course, without encephalopathy. Thus, MS is an unlikely diagnosis for the previously described clinicoradiologic findings seen in this patient.
Encephalitis patients have variable clinical presentations including fever, headaches, focal neurologic deficits, seizures, and altered level of consciousness. Cerebrospinal fluid examination can aid in the diagnosis of encephalitis, which can demonstrate pleocytosis and elevated protein levels. Polymerase chain reaction can confirm infectious origin of encephalitis.
Clinical presentation and imaging findings also vary according to the causative agent. Herpes viruses are the most common cause of viral encephalitis. Neonatal herpes encephalitis is usually caused by the herpes simplex type 1 (HSV-1). Childhood and adult herpes encephalitis is usually caused by HSV-2. HSV-2 encephalitis has quite typical imaging presentation. Parenchymal changes commonly involve the medial temporal lobes, the insular cortex, and the basifrontal regions. Encephalitis can be a good explanation for this patient’s symptoms. However, when imaging findings are taken into account, the inconsistent pattern associated with the most common forms of encephalitis was notably absent, excluding encephalitis as a diagnosis.
Acute disseminated encephalomyelitis is typically a monophasic entity, which can accompany encephalopathy. Its pathophysiology encompasses inflammation and demyelination of the white matter, which is usually bilateral and asymmetric, typically succeeding a viral infection or vaccination. Acute disseminated encephalomyelitis not only involves the white matter, but the basal ganglia, less frequently the thalami show changes on imaging. The absence of viral infection or recent vaccination excludes the possibility of ADEM. It is important to stress that CC lesions in MS and ADEM are typically located in the inferior-most portions of the CC, whereas in cases of SS, lesions are centrally located.
Susac syndrome is a rare type of microvascular disease that affects the brain, retina, and cochlea. The disease consists of a clinical triad of acute/subacute CNS dysfunction (encephalopathy), sensorineural hearing loss, and branch retinal artery occlusion (BRAO). Susac syndrome typically affects young females around the second to fourth decades (mean age of 31.6 years), with a male-to-female ratio of 1:3.5.
The pathogenesis of SS is not fully known. Evidence suggests that it consists of an immune-mediated endothelial disease, which may be caused by anti-endothelial cell antibodies, that subsequently causes small vessel occlusions in the brain, retina, and inner ear.
It is important to stress that less than 15% of patients present with the complete clinical triad at disease onset: the average delay for patients to complete development of all triad symptoms is approximately 21 weeks. Presumably, the major reason SS is misdiagnosed originates from the presumption that the presence of the entire triad is mandatory for a definitive diagnosis. At disease onset, the majority of symptoms are CNS-related or hearing loss. Patients usually complain of headache, which may routinely coexist with encephalopathy; memory, concentration, and executive function impairment; confusion; and disorientation. Susac syndrome diagnosis relies mainly on the tripod of MRI, RFA, and audiometry.
Magnetic resonance imaging of the brain is the imaging modality of choice to diagnose SS. The reported prevalence of CC involvement varies in the literature, from 78% to 100% of patients. Any part of the CC can be involved; however, the central callosal fibers are particularly more susceptible, sparing the periphery. Corpus callosus lesions usually measure 3 to 7 mm and are thought to be due to occlusion of small, precapillary arterioles (< 100 μm). Linear T2 hyperintense defects and rather large, round lesions (also known as snowballs) may also be demonstrated. Multiple T2 hyperintense lesions may also involve the periventricular white matter, basal ganglia, cerebellum, subcortical white matter, centrum semiovale, thalamus, cerebellar peduncles, and brainstem. Appearance of the characteristic string-of-pearls in the external capsule is another useful indicator, always associated with CC lesions. Frequently, leptomeningeal enhancement is present (30% of the cases), in the acute phase and may show abnormal signal on DWI. Diffuse volume loss may be noted in chronic staged SS.
These typical CC lesions should prompt retinal examination for BRAO, best demonstrated by RFA. Gass plaques (yellow-white deposits and plaques distant from retinal arteriolar bifurcations) are frequently present and reflect endothelial damage. Ophthalmic manifestations of SS are the result of multiple, bilateral BRAO, leading to segmental loss in the visual fields of one or both eyes. Vision loss can be extensive or subtle, depending on the number and site; many patients may not complain about visual field loss.
Hearing abnormalities are typically bilateral and asymmetric, with abrupt onset, and may even develop overnight. Hearing impairment may also be accompanied by tinnitus, vertigo, nausea, vomiting, nystagmus, and unsteady gait. Like BRAO, hearing loss may be difficult to evaluate in the encephalopathic patient. Brainstem auditory evoked response may show loss of wave I (cochlear response). Audiometry may show that the lower tones are first affected, reflecting the vulnerability of the cochlear apex to micro-infarction.
No consistent laboratory abnormalities have been found. Sedimentation rate and C-reactive protein levels may be elevated. Cerebrospinal fluid examination may show lymphocytic pleocytosis, and protein levels are invariably elevated from encephalopathy. During the encephalopathy stage, EEG will show diffuse slowing. Cerebral arteriography is normal, since the precapillary arteriole is below the resolution of arteriography. Elevation of factor VIII and von Willebrand factor antigen levels may occur, probably due to endothelial perturbation. Brain biopsy may be performed, which can show micro-infarctions and endothelial changes that are typical for anti-endothelial cell injury syndromes.
Susac syndrome treatment is based mainly on immunosuppression. Steroid therapy is the pillar of treatment, and is most effective in severe cases when used in conjunction with intravenous immunoglobulin and cyclophosphamide. While there is a natural tendency for the disease to improve spontaneously, there are catastrophic cases that progress dramatically to severe dementia in a relatively short period.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree