26 Percutaneous Enteral Access
Jonathan M. Lorenz
26.1 Introduction
Percutaneous enteral access (PEA) devices are key components in the management of a wide variety of conditions requiring long-term enteral nutrition, gastroenteric decompression, and gastroenteric diversion. PEA devices, which are placed routinely by radiologists, gastroenterologists, and surgeons, include percutaneous gastrostomy tubes (pGTs), gastrojejunostomy tubes (pGJTs), and jejunostomy tubes (pJTs). Given the ease of placement of PEA devices using minimally invasive options, it is not surprising that their use has steadily increased. 1 This chapter reviews the indications for PEA device placement, the devices that are available, placement techniques, aftercare, and the management of complications related to these devices.
Enteric access options include temporary access through bloodless anatomic pathways if the device is required for less than 4 to 6 weeks and permanent access through a percutaneous pathway if the device is required for a longer interval. 2 Temporary options include nasally or orally placed tubes terminating in the stomach, duodenum, or jejunum. Minimally invasive permanent options are the focus of this chapter and include pGTs, pGJTs, and pJTs placed endoscopically, usually by a gastroenterologist, or with imaging guidance, usually by a radiologist. In terms of nomenclature, recommendations from the Society of Interventional Radiology (SIR) Standards of Practice guidelines will be followed in this chapter. 3 The terms “transoral” (also called “pull-type”) placement and “transabdominal” (also called “push-type”) placement will be used to describe the route of tube advancement through the abdominal wall. As a given provider may be skilled in a variety of placement techniques, specific medical disciplines will be replaced by the general terms “imaging-guided” and “endoscopic” when describing techniques of placement.
26.2 Indications
In general, indications for PEA include long-term enteral nutrition, gastroenteric decompression, and gastroenteric diversion. The latter two indications are widely accepted, but controversy exists regarding the possible overuse of percutaneous enteral nutrition. 4 For some conditions, published results show that the benefits clearly outweigh the risks, but for others, PEA is commonly used despite no clear benefit.
For the decompression of malignant and nonmalignant gastrointestinal (GI) obstruction, PEA is an accepted, standard option with measurable benefits. 5 , 6 , 7 Depending on the clinical presentation and indication for device placement, this application can improve quality of life (QoL), improve survival, prevent immediate morbidity and mortality, and obviate the need for surgical intervention. 8 Examples include definitive treatment of postoperative abdominal adhesions and palliative decompression of cancers such as gynecological malignancies. 5 , 6 , 7 , 9 Another well-established application is the diversion of GI contents and formula from a benign or malignant esophageal, gastric, or intestinal fistula to promote healing and to facilitate enteral feeding.
For functional impairment or mechanical obstruction of the upper GI tract that prevents swallowing, PEA for enteral nutrition improves QoL and survival for select indications. 10 Benign examples include esophageal strictures and neurological conditions such as stroke, traumatic brain injury, amyotrophic lateral sclerosis, and neuromuscular disorders in children. Malignant examples include head and neck and esophageal cancer, although the use of PEA devices in patients with these conditions has been associated with a higher procedural risk. 11 In some cases, impairment in swallowing results in aspiration pneumonia; published studies have reported mixed results with PEA devices used to prevent this condition in high-risk patients. 12 , 13 Some research found that PEA devices offer no clinical benefit in preventing aspiration pneumonia, and PEA itself can be complicated by aspiration both during placement and during feeding. 14 For patients with other indications for PEA and documented aspiration pneumonia, conversion from a pGT to a pGJT or pJT may mitigate or prevent this complication. 15
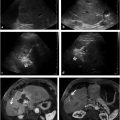
For select chronic conditions that cause failure to thrive, PEA can improve QoL and reduce morbidity. Examples include Crohn′s disease in children, metabolic disorders, and benign anatomic causes for food intolerance such as superior mesenteric artery syndrome (▶ Fig. 26.1). 16 On the other hand, when failure to thrive results from anorexia, published results argue in favor of prioritizing medical and psychiatric therapies with an occasional application of PEA. 17 Furthermore, little clinical benefit has been demonstrated with PEA for impaired feeding or failure to thrive associated with cachexia caused by terminal cancer with a poor prognosis for long-term survival, dementia-related loss of cognitive function, or permanent vegetative states. 18 , 19 Patients with poor or absent cognitive function may lack the ability for self-determination, and the decision by caregivers to place PEA devices in such cases is controversial as this procedure may actually prolong suffering rather than improve QoL. In many of these cases, nonmedical influences such as medicolegal pressure, input from family members and caregivers, and instructions left by patients in living wills win out over published evidence, leading to the placement of a PEA device.

For most indications, pGT is the most commonly used PEA device as this type is easy to place and replace, comes in a variety of models to fit most access needs, and has a decades-long track record of technical and clinical success. However, clinical and anatomic considerations may indicate the need for additional or separate small bowel access via a pGJT or pJT. Small bowel access may be indicated to prevent vomiting and aspiration pneumonia in patients with other indications for PEA. Examples include high-risk patients with a history of gastroesophageal reflux and patients on ventilators requiring enteral nutrition. Published meta-analyses support this indication. 15 , 20 , 21 Other indications for small bowel access include extension of PEA beyond a GI fistula or obstruction for enteral nutrition, decompression, or diversion. Small bowel PEA facilitates delivery of medications and formula distal to the fistula. In such cases, a dual-lumen pGJT is helpful to allow gastric suction concurrently with small bowel feeding.
26.3 Placement Options
Imaging-guided PEA device placement requires no endoscope and instead makes use of fluoroscopy and occasionally ultrasound (US) and computed tomography (CT). Standard imaging-guided PEA requires initial advancement of a small nasogastric catheter to facilitate gastric insufflation for percutaneous puncture, but initial gastric puncture using a skinny needle for insufflation can bypass this requirement. 22 Endoscopic PEA requires the patient to be a candidate for endoscopy and to be thin enough to allow successful transillumination of the abdominal wall to guide gastric puncture. Most published reports show no difference in success and complication rates for endoscopic and imaging-guided PEA. 9 , 23 , 24 In addition, minimally invasive options are associated with comparable success rates and lower complication rates compared to surgical options 25 , 26 , 27 and can be performed under conscious sedation rather than general anesthesia in most cases. Therefore, depending on local expertise, surgical PEA device placement is usually reserved as a secondary option when other options fail or are infeasible. A major advantage of imaging-guided PEA is the ability to place tubes when endoscopic PEA fails or is infeasible, as in patients with severe facial and neck fractures, esophageal strictures, severe mucositis, head and neck tumors, or morbid obesity that prevents endoscopic transillumination (see “Techniques of Placement”). In addition, imaging-guided PEA avoids the complications of endoscopy itself such as esophageal perforation. On the other hand, advantages of endoscopic over imaging-guided PEA include bedside placement in immobile patients, the absence of exposure to ionizing radiation, and the ability to directly visualize GI pathology that may alter or contraindicate the procedure such as peptic ulcer disease and varices. Ultimately, both of these placement options are viable in most cases; in select cases, clinical and anatomic considerations may determine the appropriateness of imaging-guided, endoscopic, or surgical PEA.
In terms of the route of advancement through the skin, two basic options exist: transoral PEA, which involves pulling the tube from the mouth through the abdominal wall, and transabdominal PEA, which involves pushing the tube directly through the abdominal wall (see “Techniques of Placement”). The main advantage of transabdominal over transoral PEA is the option for PEA even when conditions prevent the advancement of large-bore, bumper-retained tubes through the upper GI tract. Examples include patients with esophageal strictures or bulky head and neck tumors. Head and neck cancer is a relative contraindication to transoral PEA because of published reports of metastatic seeding of the stomal site resulting from cancer cells that adhere to the tube as it traverses the region of the primary tumor (▶ Fig. 26.2). 28 The only obstacle to transabdominal PEA is the lack of a safe window for gastric puncture. The main advantage of transoral over transabdominal PEA is the routine placement of tubes that are large bore and bumper retained, leading to fewer long-term complications such as clogging and dislodgment. 29 , 30 For the two advancement routes, procedure-related complication rates are similar. 9 , 23 , 24 , 29

In some cases, surgical PEA may be preferable to minimally invasive alternatives. Recurrent large-volume ascites impedes stomal tract formation and is associated with peristomal leakage of ascites when minimally invasive methods are attempted; surgical gastropexy may be preferable in such cases. Interposed colon or liver during attempts at imaging-guided or endoscopic PEA may necessitate surgical PEA. In some cases, congenital or acquired conditions profoundly affect the position of the stomach and adjacent structures and may necessitate surgical PEA. Examples include extensive gastric pathology, partial gastrectomy, and gastric bypass.
26.4 Device Options
26.4.1 Small-Bore versus Large-Bore Devices
PEA devices are distinguished by two key factors: diameter and type of internal retainer. Regarding diameter, tubes less than or equal to 14 French are considered small bore. Debate exists among authors regarding placement of small-bore versus large-bore devices. For most enteral feeding indications, large-bore tubes are just as easy to place, have similar rates of procedure-related complications, and are tolerated just as well as small-bore tubes, but with fewer long-term complications such as clogging or dislodgment. A retrospective series comparing 88 small-bore pGTs to 72 large-bore pGTs found that small-bore pGTs had more complications (17 vs. 5.6%) and were less likely to meet their feeding goal. 30 Additionally, small-bore pGTs were more prone to long-term dysfunction. Despite such results, published procedural and long-term complication rates for both small- and large-bore tubes are within acceptable limits. 3 In addition, small-bore tubes have their advantages. Small, pigtail-retained pGTs can be placed quickly with minimal or no serial dilatation, making them especially suitable for gastric decompression or for difficult cases such as pJT placement or CT-guided pGT placement.
26.4.2 Internal Retainers
Internal retainers include balloon-, locking-loop-, and Malecot-retained tubes that are placed transabdominally and bumper-retained tubes that are placed transorally. The transorally placed bumper-retained pGT, the mainstay of percutaneous endoscopic gastrostomy, has been tested for decades and has proven itself to be highly durable. 5 , 7 , 9 , 27 Unlike pigtail and balloon retainers, the bumper cannot be inadvertently “unlocked” by patients or caregivers and requires strong traction force for removal in most cases. As expected, published rates of dislodgment are low. Funaki et al 29 retrospectively reviewed long-term complication rates for bumper-retained and balloon-retained pGTs and found a significantly lower rate of dislodgment for the former. In addition, procedure-related complications such as peristomal leakage, peritoneal leakage, and pneumoperitoneum are uncommon with bumper-retained tubes as the use of oversized peel-away sheaths and serial dilatation is unnecessary for placement. This feature makes bumper-retained pGTs suitable for feeding within 4 hours of placement in most cases as opposed to 12- to 24-hour delays for other devices. Unlike bumper-retained tubes, balloon-, locking-loop-, and Malecot-retained tubes are advanced transabdominally under fluoroscopic guidance. Their main advantage over bumper-retained tubes is their suitability for patients with contraindications to endoscopy or transoral advancement of large-bore tubes.
26.4.3 Low-Profile Tubes
Another variation in PEA is the low-profile tube, also called a “button” tube. Its primary benefit is to reduce tampering and dislodgment in children and high-risk adults, but patients often favor this option for its cosmetic benefit. Low-profile tubes have a small external flange that doubles as an access port, eliminating the long external tube associated with other options. Low-profile tubes are manufactured with balloon-, bumper-, and locking-loop-retainers, as well as single- or double-lumen options for gastric and jejunal access.
26.5 Screening and Preparation
26.5.1 General Considerations
A patient referred for PEA device placement should undergo initial screening for the appropriateness of the clinical indication, the optimal PEA option for the indication, and considerations that may dictate a specific technique and provider. Attention is then turned to eligibility for device placement. Contraindications vary from relative to absolute depending on the type and severity, and include uncorrectable coagulopathy, hemodynamic instability, peritonitis, bowel ischemia, marked ascites, ileus or mechanical obstruction (if for enteral nutrition), the absence of an option for long-term tube management (either by the patient or by a caregiver), and the absence of a safe window for stomal creation. Patients should be screened for the need for general anesthesia versus conscious sedation and the presence of an adequate oral airway the day before the procedure. Cessation of intake by mouth after midnight should be ordered to minimize the risk of aspiration and infection. 14
26.5.2 Infection
Infection is a relative contraindication to device placement unless the patient has the absolute contraindication of peritonitis. Fever and local infections remote from the stomal site do not preclude pGT placement; if possible, bacteremia should be treated to resolution before device placement. Ventriculoperitoneal shunt is a relative contraindication, as placement of a pGT increases the risk of shunt infection and ascending meningitis. 31 Prophylactic periprocedural antibiotics have been shown to decrease postprocedural infection rates 32 , 33 and should be chosen to cover cutaneous pathogens, although patients covered with antibiotics for other conditions usually do not require additional coverage. A meta-analysis of 11 prospective randomized trials demonstrated that prophylactic antibiotics significantly reduce peristomal infections after primary PEA placement. 32
26.5.3 Safe Needle Pathway
A history of GI infection, inflammation, or obstruction and a history of surgical procedures such as gastric resection or bypass may alter the position and relationships of abdominal structures and affect the feasibility and method of PEA device placement. In addition, a variety of congenital or acquired conditions may affect the risk and feasibility of the procedure. The main concern is gastric displacement from an acceptable puncture site or interposition of structures or pathology between the puncture site and the gastric lumen. Gastric displacement occurs with large GI hernias, intrathoracic stomachs, previous GI surgery, previous abdominal surgery with resultant disruption of the gastric ligaments, and GI malrotation. Potentially interposed normal structures include the colon, small bowel, liver, or vasculature such as gastric or mesenteric arteries and varices; pathologic structures include tumors of the abdominal wall, peritoneum, or stomach; hernias; local infections; burns; and surgical wounds. To minimize the risk of nontarget puncture, all relevant past radiologic and endoscopic studies should be reviewed, although new imaging is usually not indicated. Some providers routinely administer 100 to 200 mL oral barium contrast the night before the procedure to distinguish the colon during fluoroscopically guided gastric puncture, but this practice is optional as low complication rates have been described when this step is omitted. 29 Careful evaluation of frontal fluoroscopy at the time of gastric puncture is almost always sufficient to avoid colon puncture. Additional measures include obtaining a lateral fluoroscopic view or injecting contrast through a skinny needle along the expected stomal tract under fluoroscopic guidance to expose interposed bowel or liver. Interposed vasculature and solid structures may necessitate direct US guidance of gastric puncture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree