29 Intra-Abdominal Fluid Collections
Stephen R. Lee, Ashraf Thabet, and Peter R. Mueller
29.1 Introduction
In this chapter, the percutaneous management of intra-abdominal fluid collections will be discussed. The rationale, technique, and issues related to postprocedure care of intra-abdominal abscesses will be reviewed. Special clinical scenarios, deep pelvic collections and peripancreatic collections, will be considered. Finally, an overview of the pathophysiology enterocutaneous fistulas (ECFs), traditional management strategies, and new interventional techniques for treating ECFs will be provided.
29.2 Indications/Contraindications
Drainage of intra-abdominal fluid collections can serve three main purposes: treatment of infection, characterization of fluid, and relief of symptoms. Most commonly, interventional radiologists are asked to drain fluid collections in the setting of sepsis to provide source control. At other times, a fluid collection is incidentally discovered on imaging and sampling may be required to determine its source. This commonly occurs in the postoperative setting; in such cases, the fluid collection may represent a hematoma, urinoma, lymphocele, or abscess. Less commonly, a fluid collection may be a source of discomfort for a patient. Examples include symptomatic hepatic cysts, lymphoceles, or pancreatic pseudocysts.
Contraindications to drainage of intra-abdominal fluid collections are generally relative. The risk of not intervening must be weighed against the risk of any potential procedural complications (e.g., the risk of not achieving source control by draining an abscess in a patient with sepsis vs. the risk of bleeding complications in the setting of mild coagulopathy or recent antiplatelet medication use). Relative contraindications include uncorrectable coagulopathy, hemodynamic instability, and absence of a safe window for catheter placement.
29.3 Preprocedural Preparation and Planning
Because these procedures are typically performed with moderate conscious sedation, patients are typically instructed to take nothing by mouth for 8 hours before the procedure (or otherwise per institutional guidelines). In our own practice, a combination of an opioid (e.g., fentanyl) and a benzodiazepine (e.g., midazolam) is administered intravenously with cardiorespiratory monitoring.
The Society of Interventional Radiology (SIR) consensus guidelines provide recommendations regarding management of anticoagulant medications as well as goal laboratory values in the setting of thrombocytopenia or coagulopathy. 1 Abscess drainage is categorized as a procedure with “moderate risk of bleeding,” so an international normalized ratio (INR) of less than 1.5 and a platelet count of greater than 50,000/uL is recommended.
In the setting of a possibly infected fluid collection, antimicrobial therapy is most effective if it is initiated up to 3 hours before percutaneous drainage. 2 Most intra-abdominal abscesses are polymicrobial, so broad-spectrum antibiotics to cover gram-negative rods and anaerobes are recommended. 3
Computed tomography (CT) or ultrasound is typically used for catheter drainage of intra-abdominal fluid collections. In general, CT offers better visualization of the targeted fluid collection, especially in larger patients or for collections that are deeper in the abdomen or pelvis. For complex, loculated fluid collections, CT also offers the advantage of ensuring complete drainage after initial catheter placement and can assist the interventionalist in identifying additional fluid collections that may need to be addressed. Unlike ultrasound imaging, CT images are not degraded by air within the targeted fluid collection or in the bowel.
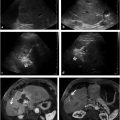
Ultrasound does offer some distinct advantages in certain circumstances. In the setting of hemodynamic instability, ultrasound can be employed at the patient′s bedside, obviating the need to transport a critically ill patient to the interventional suite and risking decompensation during the process. Draining a collection with the use of ultrasound is also typically more expedient than draining a collection with the use of CT, as catheter position is confirmed with real-time ultrasound imaging. In the pediatric population, use of ultrasound is generally strongly considered because ultrasound visualization of the targeted collection may be sufficient, obviating the need to expose the patient to ionizing radiation. Another advantage of ultrasound is that it may be performed in a traditional interventional fluoroscopy suite. This is particularly useful for complex fluid collections. Access to the collection can be achieved with ultrasound guidance; then, under fluoroscopic guidance, contrast can be injected to define the extent and configuration of the collection. Using the Seldinger technique, a wire and directional catheter can be used to navigate through the collection to ensure appropriate catheter position (▶ Fig. 29.1).

29.4 Catheter Placement Technique
There are two techniques by which a catheter can be placed into a fluid collection: tandem trocar and modified Seldinger techniques. In the tandem trocar technique, a 20- or 21-gauge needle is placed into the targeted collection under CT guidance. A small aspirate may be obtained to determine the nature of the collection. If catheter drainage is desired, the needle can then be used as a guide for catheter placement. The skin is anesthetized with local anesthesia, a skin nick is made, and the tract is bluntly dissected with Kelly forceps. The catheter is then assembled with the metal stiffener and inner trocar in place. The catheter is inserted parallel to the guiding needle to a depth determined on the planning CT. The catheter is fed off of the metal stiffener and trocar assembly, and the retention string is pulled to lock the pigtail. After the catheter position is confirmed with CT, the catheter is secured to the skin with a suture.
In the modified Seldinger technique, access may be obtained using a micropuncture technique or a larger 18-gauge needle. After confirmation of needle position with CT, a wire is passed into the collection. Wire position is confirmed with CT, and serial dilation from 8-Fr up to the catheter size is then performed in 2-Fr increments. The catheter is then placed over the wire with the internal metallic or plastic stiffener, and position is confirmed with CT. The catheter is advanced, and the retention string is pulled to lock the pigtail.
The decision regarding whether to use the tandem trocar technique or the modified Seldinger is typically based on operator preference and experience. No randomized clinical trials have compared the two techniques. However, in our experience, each technique offers distinct advantages. The tandem trocar technique is quicker and does not require wire placement and sequential tract dilation. Because there is decreased instrumentation and manipulation within the collection, there is less risk for causing sepsis. Furthermore, even when best technique is followed, each exchange of the dilator over the wire increases the chance for wire dislodgement out of the collection, particularly for small collections. The modified Seldinger technique allows for very precise catheter placement and is preferable to the trocar technique when only a small window of access is present. Also, because the wire is typically left in place before catheter deployment, the catheter can be repositioned or advanced with relative ease. A variety of catheters may be deployed with the modified Seldinger technique, including multisidehole or biliary drainage catheters, which do not typically come packaged with a sharp trocar.
29.5 Postprocedural Care
Catheter occlusion due to viscous drainage is one of the most common complications after placement of a drainage catheter. To prevent this complication, a three-way stopcock may be connected between the catheter and the drainage bag. The risk of catheter occlusion may be minimized by routinely flushing the catheter with enough volume to fill the dead space of the catheter and connecting tubing. In our practice, 5 mL of normal saline is instilled toward the catheter and 5 mL toward the drainage bag every 8 hours.
Criteria that can be used to assess the appropriateness of catheter removal include: (1) decrease in output to less than 10 mL/d, (2) absence of a residual collection on imaging (i.e., CT, ultrasound, or abscessogram), and (3) absence of significant fistula between the abscess cavity and adjacent structures that could promote redevelopment of abscess (e.g., pancreatic or biliary ducts, bowel, or bladder).
Although a decrease in catheter output may mean that the abscess cavity has collapsed, it may also suggest catheter obstruction or highly viscous collection contents. If imaging shows a residual collection and the catheter flushes with ease, this suggests that the cause of decreased output is a highly viscous collection. In this setting, tissue plasminogen activator (tPA) can be administered via the catheter into the collection. A dose of 4-mL tPA diluted in 25-mL sterile saline is administered into the catheter; the total volume instilled is smaller than the cavity volume. The catheter is clamped for 30 minutes and unclamped thereafter. This can be performed twice daily for 2 to 3 days. This protocol has been shown to be effective in reducing the need for additional catheter placement or surgical drainage. In one series of 46 abscesses refractory to initial drainage, complete evacuation of 41 abscesses (89%) was achieved after tPA was administered. 4 Notably, there were no bleeding complications directly attributable to tPA administration.
29.6 Special Considerations
29.6.1 Peripancreatic Collections
Surgical management of necrotizing pancreatitis has evolved over the past few years. 5 Minimally invasive surgical necrosectomy is now being performed in some centers with promising results. Mortality rates have been shown to be lower with the minimally invasive approach when compared to open necrosectomy (19 vs. 38%, respectively). 6 These techniques rely on a drainage catheter placed via a percutaneous retroperitoneal approach for access. During surgery, the drain is followed into the peripancreatic collection. The collection is opened, and a laparoscopic camera is inserted. The necrotic cavity is vigorously debrided with continuous lavage via two drains. 7
When placing drainage catheters for potential video-assisted retroperitoneal debridement of a peripancreatic collection, interventionalists should be mindful of a few points. The standard surgical approach for video-assisted retroperitoneal debridement is to dissect into the left retroperitoneum from a subcostal entry site along the percutaneous catheter into the region of peripancreatic necrosis. Therefore, attempts are made to place the catheter via a left retroperitoneal approach, anterior to the left kidney and posterior to the descending colon. The material drained from pancreatic necrosis is highly viscous and debris-filled. Accordingly, the potential for catheter clogging is high if the catheter selected is too small. At our institution, catheters up to 24-Fr are routinely placed. Inadvertent traversal of adjacent organs and large blood vessels by these large catheters can be avoided by noting the position of the organs and vessels on the preprocedural scan. In our practice, these catheters are typically placed using the modified Seldinger technique (▶ Fig. 29.2).

29.6.2 Deep Pelvic Collections
Deep pelvic collections can pose a challenge for even the most seasoned interventionalist. A safe percutaneous window is frequently obscured by the bowel or bladder in such cases. Review of imaging before the patient′s arrival to the interventional suite may alert the interventionalist to the need to decompress the bladder by having the patient void or by placing a urinary catheter. In general, an anterior approach is preferable if there is a safe window to the collection, as supine positioning is typically more tolerable during the procedure. Additionally, in our experience, there is less risk of dislodgement for catheters placed via an anterior approach compared to those placed via a posterior approach. If a safe anterior approach cannot be achieved, a transgluteal approach may be a good option (▶ Fig. 29.3). Inadvertent injury to the sciatic nerve and gluteal arteries can be avoided by ensuring that the course of the catheter remains close to the lateral margin of the sacrum. Transrectal and transvaginal approaches under ultrasound guidance are also options for drainage of deep pelvic collections if a percutaneous approach is not feasible. However, one of the drawbacks to these techniques is that catheter dislodgement after placement is common because of difficulty in adequately securing the catheter.

29.7 Enterocutaneous Fistulas
29.7.1 Pathophysiology/Classification
ECFs can be classified based on their anatomy, etiology, or physiology. Anatomically, an ECF is defined by the portion of the gastrointestinal (GI) tract from which it originates. ECFs most commonly arise, in descending order, from the small bowel, colon, stomach, and duodenum. ECFs can also be classified according to their volume of daily output. ECFs with an output of < 200 mL/d are classified as low output, those with an output of 200 to 500 mL/d are classified as moderate output, and those with an output of > 500 mL/d are classified as high output.
The etiologies of ECFs are variable but can be recalled with the mnemonic “FRIEND” (▶ Table 29.1). Approximately 80% of ECFs are associated with a previous surgery, most commonly surgeries related to malignancy, inflammatory bowel disease, and adhesions. Approximately 20% of ECFs are spontaneous, with no specific inciting event. The most common causes of spontaneous fistulas are malignancy and inflammatory bowel disease. Although, most ECFs occur postoperatively, interventionalists are likely to encounter spontaneous fistulas more frequently, as these patients are typically poor surgical candidates in whom conservative management is more likely to fail.
F | Foreign body |
R | Radiation |
I | Inflammation |
E | Epithelialization |
N | Neoplasia |
D | Distal obstruction |
Classification of ECFs based on their anatomy, etiology, and physiology is important, as all of these factors may provide some prognostic value with regard to spontaneous closure and overall mortality (▶ Table 29.2). 8 In one of the earliest series of patients with ECFs, Edmunds et al 9 noted an overall mortality rate of 54% in patients with high-output small bowel ECFs compared to 16% in patients with low-output colonic fistulas. The higher mortality rate of high-output fistulas was confirmed in a more recent series by Lévy et al, 10 who reported a mortality of 50 versus 26% in high- and low-output fistulas, respectively. In a series spanning 30 years and 404 patients, Soeters et al 11 also noted higher complication rates, specifically uncontrolled infection and malnutrition, with high-output small bowel fistulas. These fistulas were also less amenable to medical or surgical treatments. Although the mortality associated with high-output fistulas has decreased significantly over the past few decades, 12 the mortality differences discovered in earlier series elucidate key differences among the natural histories of different types of fistulas and help clinicians to create informed treatment strategies.
Factor | Favorable | Unfavorable |
Organ of origin | Esophageal Duodenal stump Pancreatic, biliary Jejunal Colonic | Gastric Lateral duodenal Ligament of Treitz Ileal |
Etiology | Postoperative (anastomotic leakage) Appendicitis, diverticulitis | Malignancy Inflammatory bowel disease |
Output | Low (< 200–500 mL/d) | High (> 500 mL/d) |
Fistula characteristics | Tract > 2 cm Defect < 1 cm | Tract < 1 cm Defect > 1 cm |
Nutritional status | Well-nourished Transferrin > 200 mg/dL | Malnourished Transferrin < 200 mg/dL |
Sepsis | Absent | Present |
State of bowel | Intestinal continuity Absence of obstruction | Bowel discontinuity Distal obstruction Large abscess Previous radiation |
Source: Data from Evenson and Fischer. 8 | ||
Fistulas occur in 17 to 43% of patients with Crohn′s disease (CD), 13 and ECFs associated with CD have unique features. 14 Patients with CD frequently have more than one site of active disease that needs to be addressed. The optimal medical management strategy for CD has also evolved over the past 20 years with the introduction of biological medical therapy and immunomodulation. Infliximab, a monoclonal antibody against tumor necrosis factor, has been shown to be effective in reducing the number of fistulas and ensuring a sustained response with an overall reduction in fistulizing disease. 15 , 16 This highlights the importance of a multidisciplinary approach when treating patients with ECFs related to CD, as optimal medical management is recommended before any invasive intervention. However, in spite of best medical therapy, many patients will require interventional or surgical management for fistulizing CD. In a series of 26 patients, Poritz et al 17 found that although either a partial or complete response to infliximab was seen in 61% of patients, 73% either required surgery or had abscesses remaining after medical therapy. Surgical techniques must also be adapted in patients with CD (although this is beyond the scope of this chapter). For instance, in a subgroup analysis of their series of 203 patients who underwent surgical management of ECF, Lynch et al 18 reported a higher recurrence rate in patients with CD who underwent oversewing than those who underwent resection (75 vs. 15%, respectively).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree