30 Pediatric Gastrointestinal Interventions
Shellie C. Josephs, Lisa Kang, and Kristi Bogan Oatis
30.1 Introduction
Gastrointestinal (GI) interventions in children are very similar to those performed in adults; however, in children they are performed only on a smaller scale, both in number of patients treated and in the size of the target of intervention. Diseases affecting the liver in children range from benign steatosis to portal hypertension and cirrhosis. Some focal lesions, such as infantile hepatic hemangioma (IHH) and hepatoblastoma, are unique to the pediatric population. Pediatric patients are more likely to undergo liver transplant to cure their underlying condition, and biliary and vascular complications after the transplant frequently require interventional skills for less invasive management. Interventional radiologists may additionally encounter vascular tumors and congenital shunts in this population. This chapter discusses manifestations of GI disease specific to children and presents evidence regarding interventional radiology treatment options.
30.2 General Considerations for Pediatric Interventional Procedures
Pediatric patients have an increased sensitivity to ionizing radiation compared to adults; physician awareness of this fact can promote optimal radiation safety practices. Image Gently, Step Lightly is a campaign designed to promote minimization of radiation dose in pediatric interventions, in accordance with the principle of “as low as reasonably achievable” (ALARA). 1 ▶ Table 30.1 lists measures recommended by the Image Gently, Step Lightly campaign that can be employed to reduce radiation exposure.
30.2.1 Anesthesia and Sedation
Most of the GI procedures discussed in this chapter are performed under general anesthesia to reduce patient and respiratory motion, as well as patient anxiety and pain. Anesthesiologists trained specifically in the care of children are critical to this effort, as medication dosing is weight based and a small volume of blood loss can have significant physiologic effects.
30.2.2 Fluids and Contrast
In neonates and young infants, care must be taken to minimize the volume of intravascular fluid administered, as small amounts can have a significant effect on overall fluid balance. 2 Iodinated contrast must be accounted for; careful coordination and communication between the radiologist and anesthesiologist are critical in this matter. Senthilnathan et al 3 reviewed the cases of more than 2,300 pediatric patients who underwent cardiac catheterization and concluded that adverse events, including contrast-induced nephropathy, were exceedingly rare in patients who receive iodinated contrast doses of 6 mL/kg or less. Hyponatremia and increased serum osmolality have been documented in pediatric patients undergoing cardiac catheterization who received higher doses of nonionic contrast (mean, 6.1 mL/kg). 4 The Joint Quality Improvement Guidelines by the Society of Interventional Radiology (SIR) and Society of Pediatric Radiology Interventional Committee state that contrast volume should not exceed 4 to 5 mL/kg in infants and 6 to 8 mL/kg in older children. 5 Dilution of contrast material with up to an equal volume of saline does not cause significant image degradation in small children.
30.2.3 Patient Safety
Padding of pressure spots and patient positioning are important for examinations performed under anesthesia. Hypothermia is a serious risk in the interventional suite; neonates and infants are especially susceptible. Minimizing thermal loss by limiting skin exposure, especially when the patient is wet, and providing continuous external warming are important measures for decreasing this risk. 2 Chlorhexidine gluconate/isopropyl alcohol preparations can safely be used to clean the skin with little to no absorption. Povidone iodine can be transcutaneously absorbed, especially in young infants, and is therefore not commonly used. 6
30.2.4 Arterial Access
Obtaining arterial access in pediatric patients can be challenging, especially in neonates. Children′s arteries are significantly smaller and more likely to exhibit vasospasm or arterial dissection than arteries in adults. Occlusion is also more common and may be caused by the larger ratio of catheter diameter to vessel lumen size and by the application of excessive pressure when obtaining hemostasis. In general, ultrasound (US) guidance and single-wall puncture technique are recommended. The smallest diameter catheter with which the task can be accomplished (typically 3–4 Fr) should be used. Sheaths are rarely used unless embolization is being performed. In the absence of a contraindication, systemic heparin is usually administered; doses range from 50 to 100 IU/kg depending on the estimated duration of the procedure. 5 Puncture site complications are more common in patients younger than 1 year, with an incidence of up to 7 to 10%. 7
30.3 Liver Biopsy
Over the past decade, many tertiary-care institutions have shifted toward the practice of having a dedicated pediatric interventional radiologist perform liver biopsies. 8 , 9 Imaging guidance for this procedure has been shown to be safe and cost effective 10 , 11 and has facilitated the use of outpatient biopsies so that hospital admission is no longer routinely required. Preprocedural measures used for pediatric patients, including laboratory evaluation of coagulation parameters and withholding of anticoagulant/antiplatelet medications, are the same as those used for adults, with guidelines for percutaneous image-guided biopsy published by the SIR. 12 Contraindications to both percutaneous and transjugular biopsy are also similar to those in the adult population. However, there are some pediatric-specific special considerations for percutaneous and transjugular biopsies.
30.3.1 Percutaneous Liver Biopsy
For percutaneous biopsy in small patients, the left hepatic lobe is often accessed via the subxiphoid approach. However, the left liver lobe in very small infants may be too small to accommodate the biopsy needle throw; the right lobe, from a subcostal approach, can provide the increased length and width needed for adequate sampling in these cases. Note that on sonographic images, the available liver for biopsy can appear deceptively large. The use of electronic caliper measurement is recommended in very small children when planning the needle trajectory. The intercostal approach is associated with greater postprocedural pain and is therefore reserved for cases in which other approaches are not feasible. 13
An 18-gauge cutting core biopsy needle with an approximate 20-mm throw is typically used for pediatric patients. Introducer needles are seldom used for random liver biopsies or for biopsies of easily accessible focal lesions. However, an introducer is used for the rare lesions that require computed tomography (CT) guidance, to speed up the procedure and decrease radiation dose by reducing the number of scans needed for needle positioning. Diagnostic quality samples are obtained in a high percentage of percutaneous liver biopsies, with most studies reporting rates of 98.5 to 100%. 8 , 9 , 10 , 13 , 14 Nondiagnostic results are more common if the specimen length is short (1.4 vs. 1.7 cm) as reported by Short et al in a study of over 200 liver pediatric liver biopsies. 15
30.3.2 Transjugular Liver Biopsy
Transjugular liver biopsy has been performed in infants weighing as little as 4 kg. 16 The smallest available biopsy set has a shorter guiding cannula but still uses a 7-Fr sheath, requiring a minimum vein diameter of approximately 3 to 3.5 mm. 2 In the largest series of pediatric transjugular liver biopsies reported to date, Habdank et al 17 performed 74 biopsies in 64 patients over a period of 8 years. Adequate samples were obtained in 98.6% of the procedures. The authors used simultaneous transabdominal sonography to visualize the needle path and suggested that this method could be used to decrease the risk of puncturing the liver capsule or gallbladder, especially in small children and patients with reduced liver transplants. Diagnostic quality specimens for transjugular liver biopsy were, overall, slightly lower than with percutaneous biopsy, with a rate of > 95% reported. 17 , 18
30.3.3 Complications of Liver Biopsy
Several recent pediatric-specific studies of percutaneous liver biopsies performed by interventionalists reported complications that were categorized as major or minor according to SIR guidelines for adult image-guided percutaneous biopsy. 19 Hemorrhage was the most common major complication. Major bleeding complication rates after percutaneous biopsy in children range from 0 to 4.6%. 8 , 9 , 10 , 13 , 14 , 20 Studies suggest a higher incidence of hemorrhage in infants, with major hemorrhage occurring in 4.6 to 8.7% of patients, 8 , 10 , 15 including one fatal hemorrhage in a 2.6-kg infant. 15 Lower weight, lower preprocedural hematocrit, and suspected metabolic disease have been associated with increased risk of hemorrhagic complications. 15 Interestingly, Matos et al 13 observed no increase in bleeding complications when using 16-gauge biopsy needles in a series of 513 pediatric patients. Aside from hemorrhage, other reported major complications include pneumothorax, sepsis, hemobilia, and abdominal wall pseudoaneurysm. 8 , 9
Although bleeding is also the most common complication of transjugular liver biopsy, the risk appears to be lower than with the percutaneous technique. In the series reported by Habdank et al, 17 the bleeding complication rate was reduced from 11 to 5% after the addition of transabdominal US to evaluate the needle path. The reported rate of capsular perforation is 3.5%; clinically significant bleeding has been reported in only 0.35% of cases. 18
30.4 Interventions for Portal Hypertension
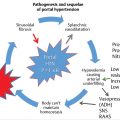
Extrahepatic portal vein occlusion (EHPVO) is the cause of portal hypertension in 70% of pediatric patients and is the most common cause of upper GI bleeding in children (▶ Fig. 30.1). 21 Thrombosis is idiopathic in up to 50% of patients with EHPVO; the remaining cases are attributed to umbilical vein catheterization, intra-abdominal infections, hypercoagulable states, dehydration, and other chronic liver disease. Cirrhosis can be a late finding in EHPVO but is more often associated with chronic cholestasis. ▶ Table 30.2 lists the potential causes of portal hypertension in this patient population. 22

Presinusoidal | Umbilical vein instrumentation |
Omphalitis | |
Congenital malformations | |
Blunt trauma | |
Intra-abdominal infections | |
Presinusoidal, intrahepatic | Congenital |
Schistosomiasis | |
Sinusoidal | Cirrhosis |
Post | Cirrhosis |
Budd-Chiari syndrome | |
Suprahepatic IVC webs | |
Venoocclusive disease | |
Cardiac disease | |
Data from Kleinman et al. 22 | |
Doppler US is a useful initial examination in the evaluation of EHPVO. This modality can be used to evaluate the extent of EHPVO, assess the general size of intrahepatic portal veins, and determine patency of the splenic and left renal veins in case a splenorenal shunt is needed.
30.4.1 Meso-Rex Bypass
The treatment of patients with EHPVO is directed toward reducing episodes of GI bleeding resulting from varices. The preferred operative intervention in children with portal hypertension related to EHPVO is meso-Rex bypass (MRB). This surgical shunt, which extends from the superior mesenteric vein to the left portal system at the Rex recess (RexR), decompresses the splanchnic circulation in a more normal physiologic route. This treatment option should be considered before complications of portal hypertension and bleeding develop. 23 A Rex vein of at least 2 mm in size is needed for adequate bypass. 24
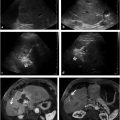
When MRB is planned, sonographic visualization of the left portal vein can be limited because of small size and low flow. Cross-sectional imaging with magnetic resonance (MR) or CT imaging is often needed for additional information (▶ Fig. 30.2a–c).

Wedged hepatic venous portography via the left hepatic vein may also be useful in evaluating the left portal vein at the RexR when this entity is not observed on other imaging studies. In one small series, Lawson et al 25 reported a sensitivity of 80% and a specificity of 100% in visualizing a vein suitable for creation of a MRB using wedged hepatic venous portography. Another small series by Chaves et al 24 demonstrated a sensitivity of 92% for identification of the left portal vein via wedged portography compared to 86 and 95% for CT angiography and MR venogram, respectively. In both series, a 4-Fr catheter was wedged into a left hepatic vein, with portography performed in the frontal, cranial oblique, and caudal oblique projections using iodinated contrast.
Shunt Revision
As with any other type of operative bypass procedure, serial follow-up examinations are needed after MRB, usually with Doppler US. Reversal of flow in the proximal intrahepatic left portal vein is a normal postoperative finding. Flow is hepatopetal through the shunt into the liver and hepatofugal in the proximal left portal vein as it crosses to the right portal vein (▶ Fig. 30.2d). Depending on the conduit used, the lumen frequently becomes smaller in caliber before the anastomosis to the left portal vein, which may cause an increase in velocity at this site. This does not represent pathologic shunt stenosis when there is a gradual increase in size of the intrahepatic portal veins and a decrease in splenic size on serial Doppler US examinations. 26 Interventions to improve or reestablish flow through a stenotic or occluded bypass have been reported, with the largest series performed by Lautz et al. 27 Nine of 15 patients (60%) were treated successfully, with 3 technical failures requiring surgical revision. Stents were placed in five of the nine patients. Stenosis was more common at the hepatic end of the shunt, likely because of the angulation and turbulent flow at this site.
In accessing the shunt via a transhepatic approach, sonographic and fluoroscopic guidance is used, similar to how this guidance is used in biliary drainage procedures, with direct puncture followed by slow retraction of the needle and contrast injection until the portal vein branches are identified. This guidance is necessary because a patent right portal vein branch target may not be readily apparent due to decreased intrahepatic portal flow. Procedural success is defined as normalization of the hemodynamic gradient. Stents are used much more conservatively in children because of issues of future growth and stent durability. The choice between a balloon-expandable stent and a self-expanding stent is often determined based on the angulation at the site of stenosis.
30.4.2 Recanalization of Chronic Portal Vein Occlusion
Scattered case reports of stent recanalization of chronic portal vein occlusion in children have been published. The first reported case by Cwikiel et al 28 described successful transhepatic access into a patent right portal vein. Transplenic access to the portal vein, with or without partial splenic artery embolization, has also been described. 29 , 30 Although there are increasing reports of transjugular intrahepatic portosystemic shunt (TIPS) placement in the adult population, the use of this technique in children is less common, likely because of the increased technical difficulties resulting from the small size of the vessels. If a child is not a candidate for an MRB and has had multiple episodes of refractory bleeding, an experienced interventionalist (with multidisciplinary consensus) may consider recanalization. Multidisciplinary discussion should include consideration of the stent location to preserve the ability to perform liver transplant in the future.
30.4.3 Transjugular Intrahepatic Portosystemic Shunts
TIPS procedures in children were first performed in 1997, with a series of 12 procedures in 9 children. 31 The original technical success rate of 78% reflects the difficulty of performing this procedure in small children. Small hepatic and portal vein size, short parenchymal tract length, and significant periportal fibrosis make this procedure technically challenging (▶ Fig. 30.3). Technical success rates of up to 100% have more recently been reported, although repeat interventions are common. 32 Bare metal stents are frequently used because of a lack of available lengths of endografts. Two recent case series described the use of the VIATORR endoprostheses in patients ranging in age from 18 months to 19 years. 33 , 34 Eight- and 10-mm diameter endoprostheses were used based on preprocedural and intraprocedural measurements of the portal and hepatic vein sizes, with initial tract dilation to 5 to 8 mm. Portosystemic gradients over 12 mm Hg determined whether further dilation was required. Technical success rates were 92 to 100%, with one failure in a patient with chronic portal vein thrombosis. Three of 23 patients developed shunt dysfunction requiring angioplasty and/or additional stent placement. In another case report, a VIATORR stent was successfully placed in a 7-month-old, 6.4-kg child despite the patient′s small size. The patient died of progression of intestinal failure–associated liver disease 2 weeks after TIPS placement without further GI bleeding. 35

Continued longitudinal growth of the child and organ growth of the liver can lead to shunt inadequacy. Regular sonographic surveillance of the TIPS is necessary, as shunt revision, including the addition of overlapping stents, is frequently required. Although placement of a VIATORR stent in a very small child may be feasible, clinicians should carefully consider the length of the uncovered segment remaining in the portal vein to ensure that this will not preclude future transplant, which is the ultimate treatment for all pediatric patients with complications of portal hypertension.
30.4.4 Partial Splenic Embolization
In children with EHPVO and portal hypertension, variceal hemorrhage is more difficult to treat when it occurs concomitantly with thrombocytopenia. The effect of TIPS placement on the spleen, including spleen-related effects on platelet function, is variable. Vo et al 33 reported an overall decrease in the mean splenic length after TIPS placement with a modest improvement in platelet count in a small series of eight patients. Di Giorgio et al 34 reported no significant change in spleen length or platelet count after TIPS placement.
Partial splenic embolization (PSE) should be considered as a means to both improve circulating platelet cell volume and, theoretically, decrease splanchnic venous flow (▶ Fig. 30.4). 36 Early reports of PSE included very high complication rates with occurrences of abscess formation, prolonged pain, and death. However, in a review of the available literature, Koconis et al 36 noted a change in the rate of reported complications over 30 years of experience with the procedure. Later reports showed that the volume of the spleen infarcted was critical to success. Embolization of too much splenic parenchyma led to more complications; embolization of too little (less than 50%) yielded a high rate of recurrent hypersplenism. 37 The goal of 60 to 70% infarcted splenic volume has been associated with the highest improvement in hematologic parameters of leukocyte count, red blood cell count, and, most importantly, platelet count. Clinical durability of this procedure at 7 and 14 years has been documented in patients with cystic fibrosis–related liver disease and hypersplenism, 38 , 39 and long-term efficacy at 5 years has been reported in 70% of children, including patients with cirrhosis from biliary atresia, EHPVO, and idiopathic causes. 40

There is no overall consensus regarding the best agent for embolization. Very small particles are not necessary, as penetration of the embolic agent to the splenic capsule is not required for infarction to occur and may in fact contribute to increased pain. Still, particles no larger than 600 to 800 μm are recommended. 41 Additionally, sparing of the upper pole of the spleen may reduce the amount of diaphragmatic inflammation and associated atelectasis. 41
30.4.5 Balloon-Occluded Retrograde Transvenous Obliteration
There are few case reports describing the use of balloon-occluded retrograde transvenous obliteration (BRTO) in children for bleeding related to gastric varices, likely because of the prevalence of EHPVO. The use of BRTO in patients with EHPVO should be considered very carefully, as these engorged veins may be the primary drainage source of the splanchnic circulation, and disruption of these pathways may lead to mesenteric ischemia. With cavernous transformation, careful evaluation of the flow to the liver and assessment of the risks are needed before this intervention is performed. 30 , 42
Sodium tetradecyl sulfate (STS) 3% is the most commonly used sclerosant in the United States because of its availability. 43 Adverse effects of STS 3% in doses exceeding 0.5 mL/kg have been reported in children who underwent treatment of vascular malformations. Complications included hemoglobinuria, oliguria, 44 and renal failure. 45 Mason et al 46 also documented alterations in coagulation factors, with decreased fibrinogen and platelets, when larger volumes were used.
30.5 Liver Transplant Interventions
There is a higher reported rate of posttransplant complications in the pediatric population. Causes of complications are widely varied but are believed to center around technical difficulties due to donor–recipient size differences, smaller caliber recipient vessels, and a small operative field. 47 , 48 , 49 , 50 , 51 Patient and organ growth is also cited as factor complicating pediatric liver transplants. 48 , 49 For both the early detection and management of complications, detailed familiarity with the type of liver graft transplanted is needed. For radiologic detection of early complications, a Doppler US examination has become the primary tool to establish a baseline and screen for complications. US has been shown to detect arterial complications before any elevation in liver enzymes or bilirubin occurs. 52 Contrast-enhanced MR and MR cholangiopancreatography (MRCP) imaging can be used when US imaging is equivocal or technically inadequate. 53
30.5.1 Hepatic Arterial Complications
Historically, the most common posttransplant complication affecting pediatric liver transplants was hepatic artery thrombosis (HAT), occurring in up to 40% of cases. With the use of newer microsurgical techniques and more routine use of antiplatelet agents, the incidence of HAT has fallen to less than 5%. 47 , 53 , 54 , 55 Early HAT is defined as HAT occurring within the first month after transplant, with peak incidence in the first 2 weeks. 56 Doppler US findings of HAT can be subtle. Arterial collateral formation is robust in children. Detection of collateral flow within the liver parenchyma can be falsely reassuring; in such cases, confirmatory imaging with CT angiography (CTA) or angiography is useful (▶ Fig. 30.5a,b). Unfortunately, collateral flow is insufficient to prevent biliary necrosis and graft failure in many cases. 53 , 54 If HAT is detected early, revascularization can improve 20-year graft survival from 24 to 77%. 57 The immediate choice of surgical or interventional treatment with thrombolysis varies among institutions. Early thrombolysis has been successfully performed in infants as young as 6 months and as early as 4 days after transplant and may be used in conjunction with angioplasty and possibly stenting. 52 , 58 Dosing of thrombolytics is not well documented, and there are no formal studies assessing the use of tissue plasminogen activator in pediatric patients. A median dose of 0.3 mg/kg/h for arterial thrombolysis has been reported in the literature; however, major bleeding occurred in 11% of patients and minor bleeding occurred in 43% of patients. 59 Lower dose protocols of 0.03 to 0.1 mg/kg/h have been shown to be effective in venous thrombolysis in children. 59

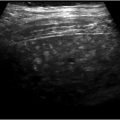
Hepatic artery stenosis at the surgical anastomosis plagues the pediatric population and has become more common than HAT. Doppler US detection of this complication is challenging in a small child, but low resistive indices (< 0.5), delayed acceleration times (> 80 ms), and tardus parvus waveforms within the intrahepatic arteries (▶ Fig. 30.5c–f) should prompt close monitoring or further workup. 53 , 60 Increased systolic velocities at the anastomotic site within the first 72 hours can be normal, and serial monitoring with Doppler US can help differentiate elevated velocities from postoperative edema. 60
Treatment with angioplasty and reserved stent placement in pediatric patients is similar to treatment in the adult population, with refractory cases requiring surgical revision. 47 , 60 Pre-procedural initiation of aspirin therapy is recommended. The greatest difference between the adult and pediatric populations is the small size of the pediatric artery. Short length (1–1.5 cm) coronary angioplasty balloons with diameters ranging from 2 to 5 mm are usually required in pediatric patients. Arterial spasm is very common and should be pretreated with either nitroglycerine 61 or a calcium channel blocker, usually intra-arterial verapamil. The largest reported series of stent placements in pediatric patients included seven children aged 5 to 16 years treated with a combination of stent types, including two covered stents for angioplasty-induced rupture. Five patients survived, and stent patency was documented in four of these patients on follow-up at 9 to 40 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree