5
Pediatric Imaging
Ethan A. Smith • Wilbur L. Smith
Ask any pediatrician and they will tell you: Children are not just little adults. Sure, the body parts (hearts, eyes, noses) are the same, but the fact that children are constantly growing and changing subjects them to different diseases as well as different radiographic appearances. As children evolve from young neonates to adult-sized (but not necessarily adult brained!) adolescents, the changing proportions and appearances of the various structures can be confusing.
The classic illustrative example of this is concept is the thymus, that ubiquitous but often misinterpreted anterior mediastinal “mass” seen on chest radiographs in young children (Fig. 5.1). This organ, important in the immune response, usually becomes inconspicuous by age 5 or so; however, its appearance and size are both extremely variable. It is not uncommon to find thymic tissue on chest computed tomography (CT) scans in patients up to age 20 years (Fig. 5.2). The thymus is a living piece of tissue that changes its configuration in a number of ways. In response to stress, it may shrink; after the stress has abated, it may grow (so-called “thymic rebound”). When indented by the ribs, it may form a wavy border; in pathologic conditions, such as a pneumomediastinum, it may even be displaced superiorly and laterally over the lung fields. With this variability in mind, the first rule in looking at children’s radiographs is to expect change and variation and consider those factors before inventing pathology that is not real (Fig. 5.3).
In general, congenital abnormalities are much more likely to present as clinical problems in neonates and younger children than they are in adults. Basically, if a patient gets to adulthood without a congenital anomaly bothering them, it is unlikely to cause clinical problems (i.e., it may just be a normal variant as opposed to a true disease). When you deal with an abnormal abdomen in a neonate, a congenital anomaly is extremely likely; in a 4-year-old it is somewhat likely; and in a 15-year-old it is less likely. If you play the odds, in an 80-year-old you probably should not even think of a congenital abnormality as the cause of an acute abdomen. Having said this, I know that everyone will be able to find the unusual case of a congenital defect causing grief to an 80-year-old, but remember that it is the zebra, not the horse!

FIGURE 5.1. The triangular opacity (arrow) arising from the mediastinum in this healthy 3-month-old is a typical appearance of the normal thymus. The triangular shape is sometimes referred to as the “sail sign.”
In this chapter we will discuss some of the common radiographic diagnoses of children, first neonates and infants, then the older children. The intent is not to be comprehensive, as there are 1,000-page tomes for that; rather, this is intended to show practical imaging approaches to common diagnoses.
CHEST: NEONATE AND INFANT
The newborn chest radiograph is a complex study with a substantial number of differences from that of an adult (Fig. 5.4). Along with our confusing friend the thymus come a variety of other factors that can make newborn chest radiographs difficult to interpret. The respiratory rate of a neonate is quite fast compared to an adult, and a neonate cannot be expected to suspend respirations while we get a nice picture; therefore, radiographs are often inadvertently obtained in expiration, resulting in lower lung volumes, crowding of structures, and even patchy atelectasis. The small size of the neonatal chest also makes things more difficult because even normal structures are very close together and are often difficult to distinguish clearly from adjacent structures.
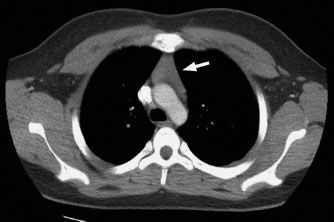
FIGURE 5.2. This 17-year-old boy had a CT scan of the chest as routine follow-up for a prior history of cancer. The triangular soft tissue in the anterior mediastinum (arrow) represents normal residual thymus.

FIGURE 5.3. The large soft tissue structure (arrows) in the superior and anterior mediastinum of this 1-month-old is all due to normal thymus. If you call anything in the anterior superior mediastinum of a neonate normal thymus, you will be right 99% of the time.

FIGURE 5.4. Normal chest radiograph of a neonate. Notice that due to the small size of the chest, the heart looks relatively prominent.
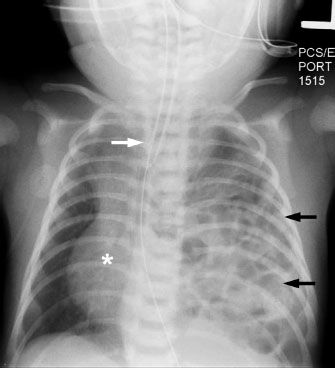
FIGURE 5.5. In this neonate with a congenital diaphragmatic hernia, the entire left hemithorax is filled with bowel loops, some of which contain bowel gas (black arrows). Notice how the mass effect from the bowel loops pushes the heart (asterisk) and mediastinal structures (white arrow) toward the right, away from the side of the hernia.
Neonatal and infant chest diseases can be roughly divided into two categories: Medical and surgical conditions. Medical conditions are usually diffuse processes and require medical management. Surgical diseases can be roughly defined as anything that needs prompt intervention. By this definition, for example, a tension pneumothorax needing treatment with a chest tube is a surgical disease. In assessing a newborn’s chest radiograph when surgical disease is suspected, you must take two steps. First, identify which side is the more abnormal (most surgical conditions are unilateral). Second, determine the direction of shift of the mediastinum. This is best done by looking at the trachea, but the position of the heart and thymus can also be secondary clues. As a general (99%) rule, surgical conditions will displace the mediastinum away from the abnormal side. For example, in the instance of a diaphragmatic hernia (a condition owing to an in utero defect that allows the abdominal contents to protrude into the chest), the heart and mediastinum are clearly shifted away from the side of the hernia by the mass of the protruding bowel (Fig. 5.5).
Medical Diseases
Transient Tachypnea
All babies have to change from an intrauterine environment where their lungs are fluid filled to one where they are breathing air. This transformation, which must occur within moments of birth, involves a complex interaction of the pulmonary lymphatics, capillary vessels, and chest compression. This normal biologic process is not always smooth. In fact, many babies, if not all, have some very short-lived tachypnea in the first minute or two after being born, owing to the vagaries of clearing their normal in utero lung fluid. The physiologic phenomenon is reflected as the pleural effusions and streaky densities seen in the lungs on radiographs taken shortly after birth.
In some otherwise normal infants, the clearance of fetal fluid causes clinically significant respiratory distress and even mild hypoxia. In general, these infants are not sick enough to require an endotracheal tube, but may require supplemental oxygen or other support. This condition, called “transient tachypnea of the newborn” (TTN), improves rapidly and resolves 24 hours after birth. Radiographs obtained in an infant with TTN will show linear interstitial opacities, streaky perihilar opacities, and small (usually bilateral) pleural effusions. The lung volumes will usually be normal (Fig. 5.6). One confounding problem is that neonatal pneumonia, a much more serious condition, can also present with respiratory distress and similar radiographic findings to transient tachypnea. In this situation, the neonatologist may be forced to treat for pneumonia even if they suspect they are dealing with TTN, because the potential consequences of an untreated pneumonia can be dire. Other, less common conditions could also present with a similar radiographic picture, including congenital lymphatic abnormalities and congenital heart disease. One radiographic clue that you may be dealing with pneumonia as opposed to TTN would be the presence of a unilateral pleural effusion (Fig. 5.7).

FIGURE 5.6. Full-term baby born by cesarean section with respiratory distress immediately after birth. The streaky perihilar opacities and small bilateral pleural effusions (arrows) are typical of transient tachypnea of the newborn. Notice that the patient is not sick enough to require an endotracheal tube.
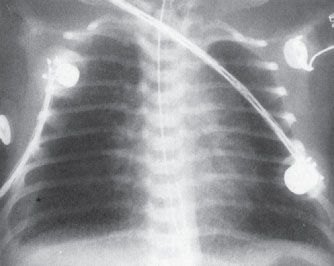
FIGURE 5.7. A very ill newborn with a streaky pattern in both lungs and a large unilateral right pleural effusion. The unilateral pleural effusion is suspicious for pneumonia. This pattern is typical of group B streptococcal infection in a neonate.
Respiratory Distress Syndrome (aka Surfactant Deficiency)
Previously known as “hyaline membrane disease,” this process is seen almost exclusively in preterm infants (except in rare instances of congenital causes). Respiratory distress syndrome (RDS) occurs as a result of deficient surfactant, the lipid-based molecule that helps to keep alveoli open by lowering the alveolar surface tension. Surfactant is produced by the type II alveolar cells and does not begin to be made in sufficient quantities until well into the third trimester. If there is insufficient surfactant, the result is diffuse alveolar collapse leading to poor oxygen exchange and respiratory compromise. Clinical information is quite helpful in the diagnosis, as these babies will be preterm and have significant respiratory distress shortly after birth, almost uniformly requiring mechanical ventilation. The radiographic appearance of RDS is secondary to diffuse microatelectasis and has four key features: (1) Low lung volumes, (2) granular opacities, (3) air bronchograms, and (4) uniform distribution throughout both lungs (Fig. 5.8). You could argue that a fifth sign, although less specific, is the presence of an endotracheal tube indicating that the infant is experiencing severe respiratory distress. With the introduction of a synthetic surfactant, the prognosis for these babies has improved tremendously. The drug is administered as an aerosol through an endotracheal tube or at bronchoscopy. The patient is moved (rolled) around in order to distribute the surfactant throughout the lungs. Occasionally in recently treated infants you may see partial clearing with residual opacities and air bronchograms at the lung bases, representing areas where the drug has not yet distributed.

FIGURE 5.8. Extremely premature infant born at 24 weeks of gestational age. This appearance is classic for respiratory distress syndrome (i.e., RDS, surfactant deficiency, hyaline membrane disease). Note the low lung volumes, granular opacities, air bronchograms, and uniform distribution. The presence of the endotracheal tube tells you that this baby is critically ill.
Meconium Aspiration
Meconium aspiration is a disease seen in term or postterm infants and occurs if the baby passes meconium while still in the womb. The sticky, tenacious meconium can then be aspirated and cause plugging and obstruction of the small airways. Postnatally, this leads to a combination of air trapping (areas of lung where air can get in but cannot get out) and atelectasis (collapsed areas where no air can get in). These infants often also have significant respiratory distress soon after birth, often requiring intubation and mechanical ventilation. Chest radiographs will demonstrate hyperinflation, coarse and patchy bilateral airspace opacities, and even areas of relative hyperlucency secondary to air trapping (Fig. 5.9). Hyperinflation can lead to alveolar rupture and pneumothorax.
A review of the radiographic features of neonatal medical lung diseases is presented in Table 5.1.
Surgical Diseases
Pneumothorax
Although pneumothorax is rare in healthy babies, neonates and infants that require mechanical ventilation (secondary to medical processes such as RDS or meconium aspiration) are at a relatively high risk (Fig. 5.10). Remember that most neonatal chest radiographs are obtained supine, so the nice, clear apical pneumothorax and pleural edge you see in an adult may be absent. Signs of a pneumothorax in a supine baby include asymmetric hyperlucency of an entire lung, deepening of the costophrenic sulcus (the “deep sulcus sign”), and sharply defined cardiac or diaphragmatic borders (secondary to these structures being outlined by air). Remember to always look for signs of tension—if you see the mediastinal structures being shifted away, or if you see an asymmetrically flattened diaphragm, you should be concerned for a tension pneumothorax which needs prompt intervention.
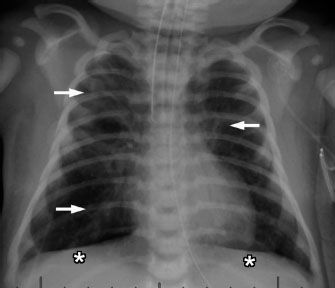
FIGURE 5.9. Neonate born at 41 weeks of gestational age with meconium aspiration. This baby is also intubated, indicated severe respiratory distress. The lungs are hyperinflated, evidenced by flattening of the diaphragm (asterisks). The patchy airspace opacities (arrows) likely represent areas of atelectasis.
Congenital Diaphragmatic Hernia
A congenital defect in the diaphragm is termed a congenital diaphragmatic hernia (CDH). If the diaphragm does not develop properly and a defect remains, bowel and abdominal contents can herniate through the hole and into the chest. The most common location for the defect is posterior and medial (a so-called “Bochdalek” hernia). CDH is also more common on the left (owing to the liver beneath the right hemidiaphragm). CDH has varying degrees of severity, depending on how big the defect in the diaphragm is and how much abdominal contents herniate through it, because the more abdominal contents there are in the chest, the less room there is for normal lung to develop. Lung hypoplasia and resultant respiratory and circulatory problems that come with it are the main cause of mortality in these patients. The radiographic appearance varies with the patient’s age. In the immediate neonatal period, the bowel loops in the chest will be filled with fluid, giving the appearance of a mass. Hours to days later, as bowel gas progresses through the loops, multiple lucent gas-filled bowel loops will be present. Due to the mass effect from the herniated abdominal contents, the mediastinal structures are often displaced toward the opposite side of the chest (see Figs. 5.5 and 5.11).
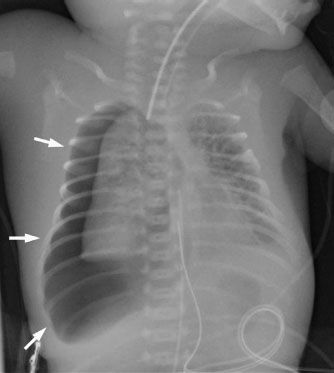
FIGURE 5.10. This premature neonate was on a ventilator due to severe respiratory distress syndrome and developed a huge right pneumothorax (arrows). The size of the pneumothorax should make you concerned for a tension pneumothorax, and this baby required an emergent chest tube. Notice that the right lung maintains a relatively normal shape despite the pneumothorax. This is due to the abnormal stiffness of the lung tissue because of the underlying lung disease.
Table 5.1

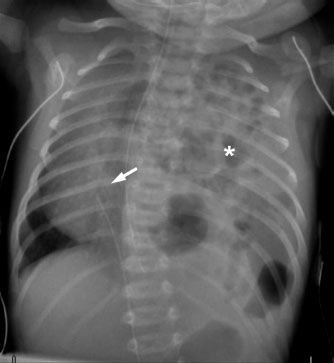
FIGURE 5.11. Another neonate with a large congenital diaphragmatic hernia. Similar to the patient in Figure 5.5, this child has a large left CDH with bowel loops filling the left hemithorax (asterisk) and shift of the heart and mediastinal structures to the right (arrow). The left lung is severely hypoplastic.
Congenital Lung Lesions
Other congenital lung masses occur infrequently, but are worth a brief mention here. The first is congenital lobar hyperinflation (CLH). This entity, formerly known as “congenital lobar emphysema,” is caused by an abnormal airway that causes a one-way valve. Essentially, air can get in to the affected segment of the lung, but it cannot get back out. This causes progressive hyperinflation of a portion of the lung, most commonly the left upper lobe. On radiographs, you will see a hyperlucent area with associated mass effect. In the immediate neonatal period, the affected lung is filled with fluid and may be opaque, but as the fluid absorbs and more air gets trapped, it will soon become hyperlucent (Fig. 5.12).
The second congenital lung mass worth mentioning is the heterogeneous spectrum of lesions that includes both congenital pulmonary airway malformation (CPAM; formerly known as congenital cystic adenomatoid malformation) and sequestration. Both of these entities involve abnormal development of a section of lung, which may or may not connect with the airways and often has abnormal vascularity. In general these lesions show up as solid masses on chest radiographs, although CPAM may occasionally be predominantly made up of air-filled cysts. Many are even identified prenatally on either ultrasound or MRI (Fig. 5.13). Both of these lesions are usually surgically resected due to a risk of recurrent infections and a small risk of future malignancy.
Esophageal Atresia
Our discussion so far has focused mostly on lung disease, but of course there are other significant organs in the pediatric chest, including the heart and the esophagus. Esophageal abnormalities that are of importance in children are usually related to esophageal atresia. The most common form of esophageal atresia is a blind-ending proximal esophagus with a fistula extending from the trachea or left main stem bronchus to the distal esophagus. Inhaled air travels through the fistula and into the rest of the GI tract; therefore, the initial films can look superficially normal, with a gas-filled loop of bowel. The clues are that the GI tract is more distended by air than usual and the proximal esophageal pouch is dilated. Clinicians become alert to this condition when the child chokes on feedings and the pediatrician cannot pass a nasogastric (NG) tube into the stomach. This also provides a clue on the chest radiographs as the NG tube will be seen within the pouch (Fig. 5.14).

FIGURE 5.12. A: A neonatal chest film shows mediastinal shift from left to right and a partially opaque fluid density in the left upper lobe. This is a patient with congenital lobar hyperinflation and only partial emptying of amniotic fluid from the abnormal part of the lung. B: The same patient at the age of 11 months demonstrates findings more typical of lobar hyperinflation with a markedly hyperinflated left upper lobe herniating across the midline (arrows).
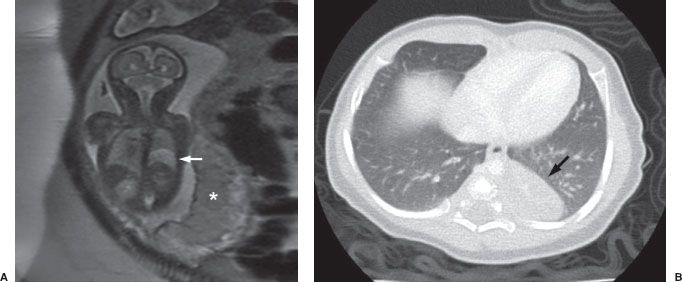
FIGURE 5.13. A: Fetal MRI obtained before birth, demonstrating an area of abnormal high signal (light gray) in the lower aspect of the left chest (arrow). Note the normal placenta (asterisk). B: A CT scan of the same patient obtained after birth demonstrates a rounded soft tissue mass in the left lower lobe (arrow) which proved to be a congenital pulmonary airway malformation (CPAM).
There are two less prevalent but still frequent variants of esophageal abnormalities. The first is esophageal atresia without fistula, in which case the abdomen is gasless because the infant cannot swallow any air to displace the fluid that is in the abdomen in utero (Fig. 5.15). Such infants are usually quite ill and need emergent surgery. The second variant is tracheoesophageal fistula without esophageal atresia. This so-called H-type fistula (or sometimes termed N-type fistula because of the diagonal course of the fistula) can be a difficult diagnosis. Unlike with esophageal atresia, an NG tube can pass into the stomach, so the diagnosis is not readily apparent to the clinician. The child may present weeks later with frequent pneumonias because each time the infant eats, some of the material goes into the lung. Whenever you have an infant with frequent and recurrent pneumonia, this entity should be considered. An esophagram with careful true lateral positioning is often necessary to confirm the diagnosis (Fig. 5.16).
ABDOMEN: NEONATE AND INFANT
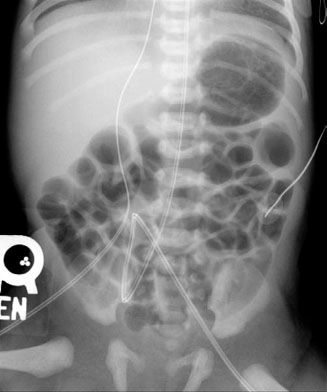
Abdominal radiographs of neonates are very different from those of adults, whereas radiographs of older children and teenagers begin to have a lot of similarities with adult. Abdominal films of neonates are especially discrepant because of a number of physiologic factors. First and foremost, neonates swallow a tremendous amount of air during their relatively inefficient breathing and eating. It is, therefore, not at all unusual to find many loops of gas-filled small bowel on the plain film of a normal neonate (Fig. 5.17), whereas in an adult or older child it is unusual to see so much small bowel gas (Fig. 5.18). In fact, it is usually abnormal and ominous to see a gasless abdomen in a neonate (Fig. 5.19)! All of this air in the small bowel makes the interpretation of the films difficult as far as determining bowel distention. The best rule to remember is that the bowel loops of a normal neonate are thin walled and lie in close proximity to each other. The appearance of thick-walled bowel or marked separation of the bowel loops suggests an abnormal intra-abdominal process (Fig. 5.20). Comparison of Figures 6.17 and 6.20 illustrates this point.
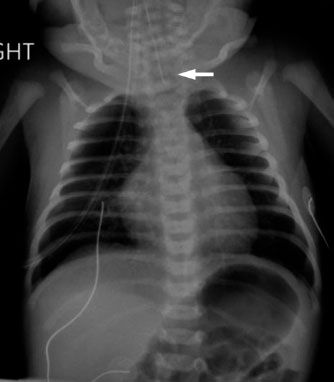
FIGURE 5.14. This baby choked on her first feeds, and the pediatrician was unable to pass a nasogastric tube. Note the tip of the nasogastric tube overlying the neck (arrow). The findings are consistent with esophageal atresia. There is bowel gas present, indicating the presence of a fistula between the trachea and the distal portion of the esophagus.
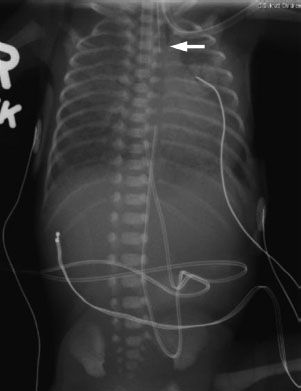
FIGURE 5.15. A premature neonate that could not tolerate feeds. The NG tube is stuck in the cervical esophagus (arrow). There is no bowel gas, consistent with esophageal atresia without a distal fistula. Notice that this baby also has uniform, granular opacities throughout both lungs, typical of respiratory distress syndrome secondary to prematurity.
The haustra of the colon are notoriously variable in their development and do not become prominent until about 6 months of age. For this reason, trying to differentiate large from small bowel on the plain radiographs of a neonate’s abdomen is usually unreliable. Occasionally, you can get lucky and be reasonably certain in differentiation; however, most of the time it is not even worth guessing. This makes the determination of whether there is rectal gas or not, even more critical. The vast majority of newborns will have gas all the way through their GI tract 24 hours after birth. If there is any doubt as to whether a child has bowel gas through to the rectum, the prone cross-table lateral film is invaluable in making this distinction (Fig. 5.21). Remember that on a supine image (which almost all neonatal abdominal radiographs are), the rectum is posterior (or dependent), whereas air collects in nondependent structures. By turning the patient prone, the rectum now becomes nondependent, so if bowel gas can get there (i.e., there is no obstruction), it will!
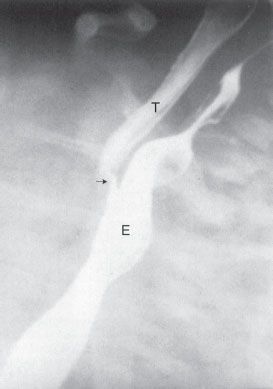
FIGURE 5.16. A barium esophagram on a baby with recurrent pneumonia shows a connection between the esophagus (E ) and the trachea (T ), a so-called H-type or N-type tracheoesophageal fistula (arrow). This abnormality can sometimes be extremely difficult to detect.
FIGURE 5.17. Normal bowel gas pattern in a neonate. While there are multiple gas-filled loops of small bowel, notice that all of the bowel loops are thin walled and are close together, not separated.
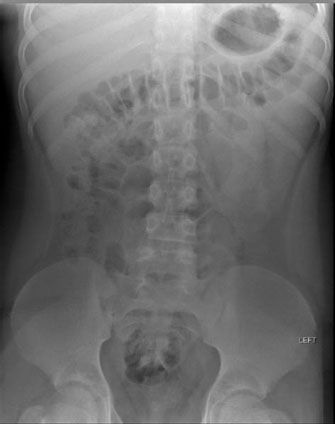
FIGURE 5.18. Normal bowel gas pattern in a 14-year-old girl. Compared to the neonate in Figure 4.17, there is much less small bowel gas. Some gas is present within the normal caliber colon, and the haustral pattern of the colon is clearly visible.
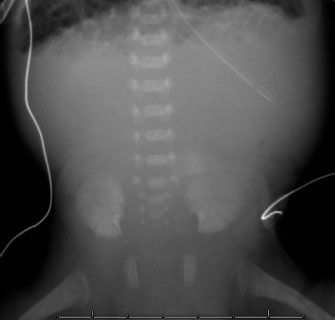
FIGURE 5.19. Completely gasless abdomen in a critically ill infant with necrotizing enterocolitis. Babies typically have at least some bowel gas, so seeing a gasless abdomen is almost always an ominous sign.
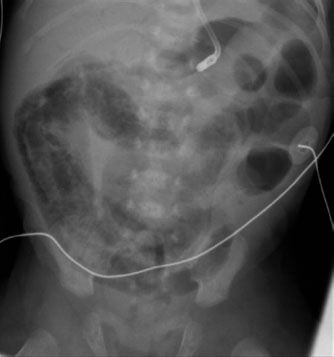
FIGURE 5.20. This is a very abnormal bowel gas pattern in another infant with necrotizing enterocolitis. Notice how the bowel loops look separated from each other. This appearance is due to thickening of the bowel walls and probably also from some free fluid within the abdomen. The linear lucencies in the bowel in the right side of the abdomen represent gas inside the bowel wall or pneumatosis, another sign that this baby is critically ill.

FIGURE 5.21. A prone cross-table lateral view of the abdomen can often be helpful in showing whether or not gas is present in the rectum.
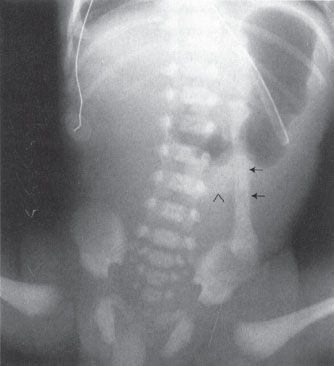
FIGURE 5.22. A newborn infant who has just begun to swallow air. Note the nasogastric tube in place in the stomach. The oblong structure to the left of the spine (arrows) almost looks like a bone of some sort; however, it is clearly attached to the umbilical stump (arrowhead ) and in fact represents an umbilical cord clamp.
A quick mention of the belly button (umbilicus, to be proper) is in order. This necessary structure and the accessories attached to it make for sometimes confusing shadows on abdominal films of neonates. Many an unsuspecting physician has called an umbilical clamp a bone or a foreign body (Fig. 5.22). The umbilicus itself protrudes much farther in a neonate than in an adult. Any coin-shaped soft tissue density lesion in the lower midabdomen of a neonate should probably be considered the umbilical remnant until proven otherwise. A good clue is that, owing to the air surrounding the protruding umbilical stump, the edges of the umbilicus are very sharply defined, particularly the inferior edge.
Neonate
The most common indication for abdominal imaging in the immediate neonatal period is the concern for obstruction. Clinically, the child will present with feeding intolerance, often with progressive abdominal distention and sometimes with failure to pass meconium. In this setting, abdominal radiographs are commonly obtained and demonstrate variable degrees of gaseous distention of the bowel. The savvy physician can use the appearance of the abdominal radiograph to occasionally make a definitive diagnosis, but more often as a guide as far as the next test is to be done. If there are only a few dilated loops of bowel confined to the upper abdomen, a more proximal obstruction should be suspected and an upper gastrointestinal fluoroscopy (an upper GI) should be performed. However, if there are multiple loops of dilated bowel throughout the abdomen, the higher-yield diagnostic test will be a contrast enema. Occasionally things are not this clear and both tests are done; however, as a general rule any neonatal obstruction suspected to be proximal to the ligament of Treitz should get an upper GI while anything more distal should start with a contrast enema.
Atresias
In a neonate, the most common cause of bowel obstruction is an atresia. Atresia occurs owing to a number of complex intrauterine processes, usually with the final common pathway of compromise of vascular supply to the wall of the bowel. Often, the affected segment completely disappears and all that is left is a wedge-shaped defect in the mesentery. The exception to this is duodenal atresia, which is caused by a failure of recanalization of the duodenal lumen. During normal development, the lumen of the duodenum is temporarily obliterated by an ingrowth of cells. If these cells then fail to regress, the lumen remains closed and the baby is left with an obstruction.
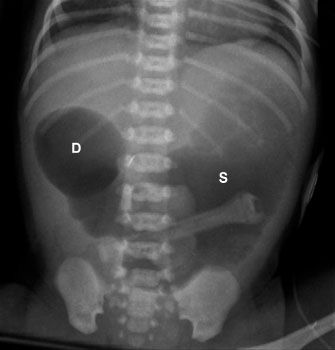
FIGURE 5.23. This newborn had marked abdominal distention shortly after birth. The abdominal radiograph demonstrates a dilated stomach (S ) and duodenal bulb (D), but no distal bowel gas. This is the classic “double bubble sign” and is diagnostic of duodenal atresia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Chapter Outline
Chapter Outline