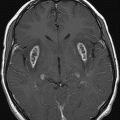
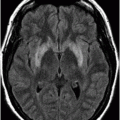
Axial T2WI at the level of the basal ganglia and thalami.
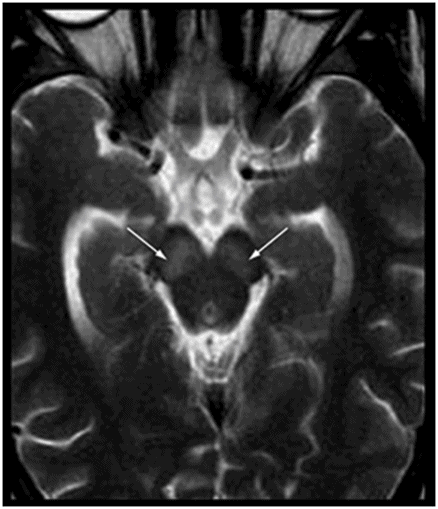
Axial T2WI at the level of the cerebral peduncles.
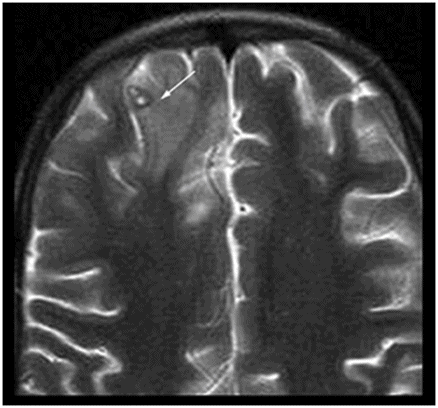
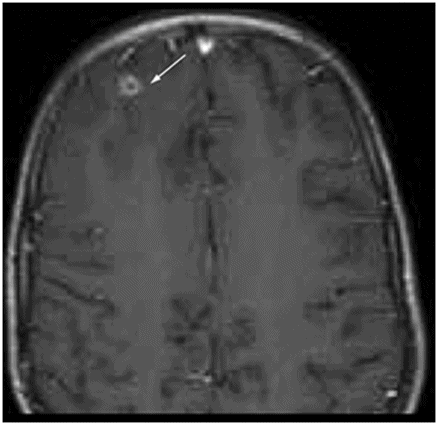
Axial T2WI at the level of the anterior frontal convexities.
Axial T1W postgadolinium image at the level of anterior frontal convexities.
Neurocysticercosis and Japanese B Encephalitis
Primary Diagnosis
Neurocysticercosis and Japanese B encephalitis
Differential Diagnoses
Cerebral malaria
Toxic-metabolic etiologies involving the basal ganglia and thalami
Paraneoplastic syndromes
Other viral encephalitides such as Epstein-Barr virus (EBV), herpes, and West Nile
Imaging Findings
Fig. 50.1: Shows nearly symmetric T2WI hyperintense signal abnormality in the bilateral basal ganglia, thalami, and substantia nigra. Fig. 50.2: Axial T2WI at the level of the cerebral peduncles and Fig. 50.3: Axial T2WI at the level of the anterior frontal convexities demonstrated a small T2 hyperintense lesion in the right frontal subcortical white matter with ring enhancement. Fig. 50.4: Axial T1W postgadolinium image demonstrates that the ring enhancement is a coexisting neurocysticercosis granuloma.
Discussion
The imaging findings overlap with several toxic-metabolic leukoencephalopathies, paraneoplastic syndromes, and a wide spectrum of viral encephalitides. High index of clinical suspicion with blood and CSF investigation is the key to diagnosing symptomatic patients residing in mosquito-infested endemic zones.
Japanese encephalitis (JE) is an endemic arboviral infection in most Asian countries with high incidence of outbreaks and prevalence, especially in India and China, with seasonal variations. It is primarily a zoonotic disease. Pigs are the most important reservoirs and amplifiers. Human infections occur by culicine mosquito bites and are incidental or dead-end host. The disease is commonly seen in pediatric and infant populations. A high index of suspicion is necessary as most cases present with febrile seizures and altered level of consciousness. Serum detection, antibody detection, and viral isolation from CSF can confirm diagnosis. High association of coexisting NCC (neurocysticercosis) and JE is known in endemic zones and is not an incidental finding. Neurocysticercosis acts as an immunomodulator, potentiates the viral virulence, and facilitates the spread of infection by altering the immune response and blood-brain barrier. The disease burden of JE tends to lateralize to the side of the brain with NCC lesions, possibly explaining the asymmetric pattern in many cases.
The prevalence rates for NCC in patients with JE are variable according to neuroimaging literature, ranging from as low as 4% to as high as approximately 19%. Mortality rates are as high as 25% with residual neurologic deficits in survivors. The virus typically involves the basal ganglia, thalamus, midbrain, hippocampus, and cerebral cortex, with unilateral or bilateral and asymmetric distribution. The lesions are hypointense on T1WI with no contrast enhancement and hyperintense on FLAIR and T2WI. Variable DWI findings have been described in the literature.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree