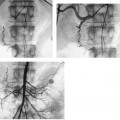
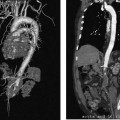
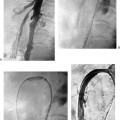
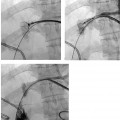
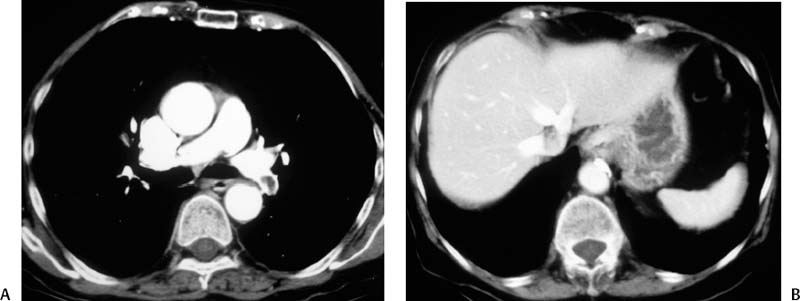
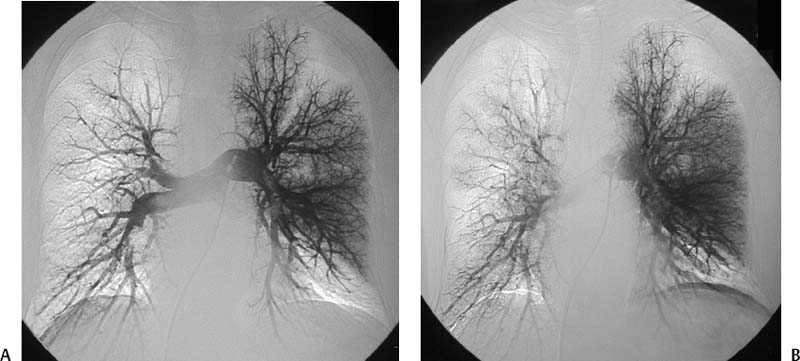
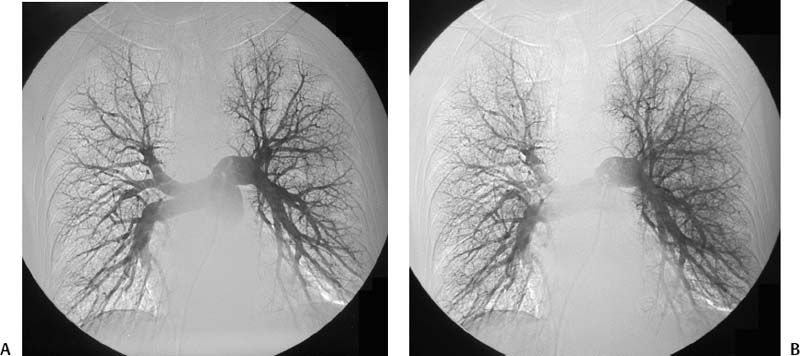
CASE 58 A 32-year-old female with a history of systemic lupus erythematosus presented to the emergency room with acute onset of severe shortness of breath, pleuritic chest pain, tachycardia, and hypotension. Figure 58-1 A 32-year-old woman with shortness of breath, chest pain, tachycardia, and hypotension. (A) Contrast-enhanced spiral CT images show large left segmental pulmonary thromboembolus. (B) Large thrombus within the intrahepatic inferior vena cava. Contrast-enhanced spiral CT showed bilateral large filling defects in the main and segmental pulmonary arteries (Fig. 58-1A). In addition, a large filling defect was present in the suprarenal inferior vena cava (Fig. 58-1B). Massive pulmonary embolism (PE) with hemodynamic collapse. As a result of the large PE, hemodynamic instability, and the low risk of bleeding complications in this young patient, she was referred to interventional radiology for emergent, catheter-directed thrombolysis of the pulmonary arteries. A Van Aman catheter was used for selective pulmonary arteriography, which confirmed the diagnosis of massive PE (Fig. 58-2). Thrombolysis was performed using 100 mg of recombinant tissue plasminogen activator (rTPA) infused over 2 hours. Marked improvement in respiratory status was noted at the conclusion of the infusion period, and the patient became normotensive. She returned to interventional radiology for catheter removal and follow-up angiogram revealed near-complete resolution of the bilateral pulmonary arterial thromboembolus (Fig. 58-3). There were no procedure-related complications. Figure 58-3 Post-thrombolysis. (A) Arterial phase pulmonary angiogram shows no residual intraluminal thrombus. (B) Delayed image shows near-uniform parenchymal perfusion. Contrast material 7F Van Aman catheter (Cook, Bloomington, Indiana) 0.035” standard guidewire (Boston Scientific, Natick, Massachusetts) Recombinant tissue plasminogen activator (100 mg; Genentech, South San Francisco, California) Each year, PE occurs in about 0.5 to 2 per 1000 people, frequently affecting hospitalized patients. The estimated death rate of undiagnosed PE is approximately 30%, as opposed to 3% when the diagnosis is made and proper treatment is provided. A wide range of risk factors have been demonstrated, including an extended period of immobility, dehydration, pregnancy and childbirth, and conditions that increase the tendency to clot blood, such as malignancy, blood dyscrasias, and the use of some drugs such as oral contraceptives. Clinical presentation is based on the respiratory and hemodynamic consequences of the underlying thromboembolus. Patients may present with a spectrum of findings ranging from sudden onset of cough, mild chest pain, dyspnea, syncope, and hemoptysis to severe hemodynamic changes such as tachycardia and hypotension, indicating right heart failure. In the last decade, an evolution in the imaging work-up of PE has resulted from technical advancements in the available imaging modalities. Ventilation/perfusion scanning in nuclear medicine was once the mainstay screening exam for PE, but this technique was largely replaced by spiral CT. More recently multidetector row CT (MDCT) has shown tremendous promise in improving the detection of peripheral emboli by providing superior resolution in the setting of diminished motion artifact. Just as the diagnostic work-up is evolving for patients with PE, so, too, is the treatment algorithm. The goal of the pulmonologist is to distinguish between patients with mild symptoms of PE and patients with life-threatening hemodynamic instability, apply the appropriate therapy in a precipitous manner, and thereby prevent escalation of the clinical symptoms. Death can result from massive PE, recurrent PE, or progression of an existing PE, and is often caused by right ventricular failure. Therefore, the goals of existing medical therapies include prevention of clot propagation (anticoagulation), prevention of recurrence (caval filtration), and restoration of hemodynamic stability in patients with massive PE (thrombolysis). In most patients with hemodynamic collapse and massive PE, death is likely to occur within 2 hours, and rapid infusion of a massive dose of rTPA is absolutely indicated despite the risks of bleeding complications.
Clinical Presentation
Radiologic Studies
Diagnosis
Treatment
Equipment
Discussion
Background
Noninvasive Imaging Work-up
CHEST RADIOGRAPH
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree