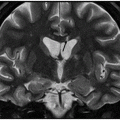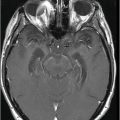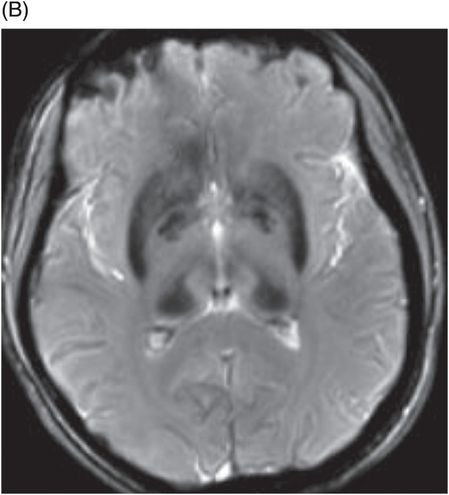
(A) Axial T2WI through the level of cerebral peduncles. (B) Axial T2WI through the level of the thalami.
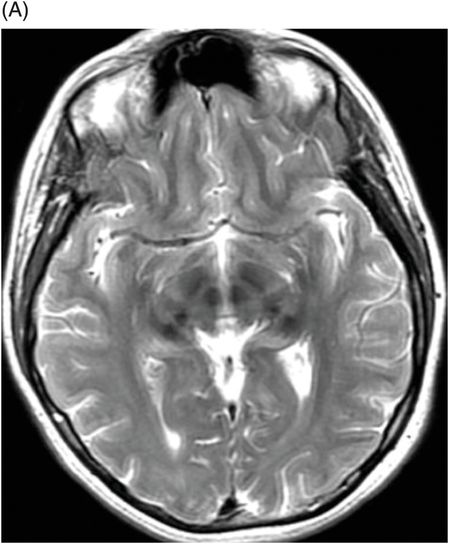
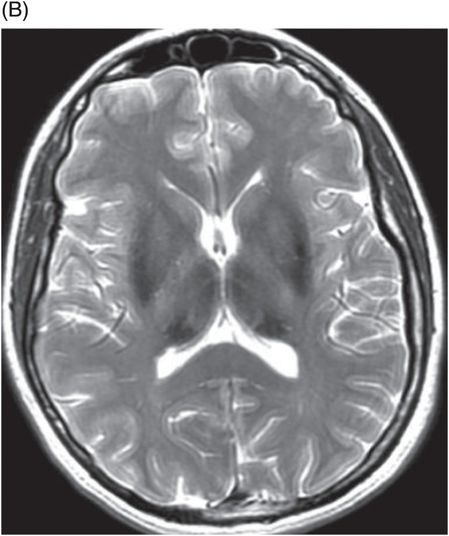
Aceruloplasminemia
Primary Diagnosis
Aceruloplasminemia
Differential Diagnoses
Neuroferritinopathy
Familial idiopathic basal ganglia calcification (FIBGC)
Physiologic calcification
Altered calcium metabolism
Hemochromatosis
Imaging Findings
Fig. 6.1: (A) Axial GRE image through the level of cerebral peduncles demonstrated hypointensity in the bilateral substantia nigra, suggesting excessive iron deposition, and (B) Axial GRE image through the level of the thalami demonstrated extensive iron deposition involving bilateral basal ganglia and thalami. Fig. 6.2: (A) Axial T2WI through the level of cerebral peduncles demonstrated hypointensity in the bilateral substantia nigra and red nuclei, suggesting excessive iron deposition, and (B) Axial T2WI through the level of the thalami demonstrating hypointensity, secondary to extensive iron deposition involving bilateral basal ganglia and thalami.
Discussion
Early-onset movement disorders and neurocognitive decline are indicative of metabolic diseases with CNS involvement. In this patient, MRI of the brain demonstrated abnormal iron accumulation in the deep gray nuclei. In addition to the CNS manifestations, she also has diabetes and microcytic anemia. Similar presentations are seen in both neuroferritinopathy (NFT) and aceruloplasminemia (ACP). However, NFT has microcytic anemia that is responsive to iron supplementation. In addition, in NFT, serum ferritin level is low, not high. High serum ferritin and low ceruloplasmin levels are diagnostic of ACP. Typically, patients with ACP develop visual problems due to retinal degeneration. This patient also developed retinal degeneration that further supports the diagnosis of ACP.
Patients with extensive calcification of deep gray nuclei, such as FIBGC, and patients with altered calcium metabolism, such as hypo- and hyperparathyroidism, pseudohypoparathyroidism, or renal failure, may demonstrate extensive signal loss on GRE and SWI sequences. Of all these, movement disorders are prevalent only in patients with FIBGC. In physiologic calcification, the extent of calcification is not as severe as with these conditions and abnormal serum chemistry is not present.
Aceruloplasminemia is one of the two known, adult-onset neurodegeneration with brain iron accumulation (NBIA) subtypes (the other one is NFT) and is caused by mutation in the ceruloplasmin gene located in chromosome 3q. The mutation results in the loss of ceruloplasmin protein function, the key transporter of copper, which indirectly results in altered homeostasis of systemic and CNS iron trafficking and tissue iron mobilization. This causes abnormal iron deposition in different areas of the brain such as deep gray nuclei of the basal ganglia, red nuclei, substantia nigra, dentate nuclei, thalami, cerebral cortex, and cerebellum. Deep gray nuclei and dentate nuclei are affected more severely than the cerebral cortex. Iron typically deposits in the perivascular space with degeneration of astrocytes. Enlarged and/or deformed astrocytes and spheroid-like globular structures are characteristic neuropathologic findings in ACP. In addition to the brain, abnormal iron accumulation also occurs in the retina, myocardium, pancreas, and liver.
Dementia, abnormal movements, and diabetes in a middle-aged patient are typical clinical findings of ACP. Patients classically have blepharospasm, chorea, torticollis, and ataxia. In addition to the movement disorders, patients frequently have retinal degeneration. There is also microcytic anemia that is responsive to ceruloplasmin rather than iron supplementation. Serum ceruloplasmin levels are characteristically low, in contrast to high ferritin levels.
Similar to the other NBIA subtypes, the classic imaging finding seen is abnormal excessive iron accumulation in the brain. In addition to the globus pallidus and substantia nigra, excessive iron accumulation is seen in the caudate nuclei, putamen, thalami, red nuclei, and dentate nuclei. The degree of iron deposition in patients with ACP is more extensive compared to the other NBIA subtypes. There is no cystic degeneration of the basal ganglia, a feature that can be used to differentiate ACP from NFT, as both of these entities share common clinical presentations. There may be associated cerebellar atrophy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree